Researchers Reveal Mechanism of Actions of Cholecystokinin Receptors
Cholecystokinin (CCK) and gastrin are the earliest discovered gastrointestinal hormones. They are the most abundant peptides in gastrointestinal tract and central nervous system, acting as physiologically important hormones and neurotransmitters through two CCK receptor subtypes, CCKAR and CCKBR. These two receptors engage in fundamental physiological actions such as satiety regulation, pancreatic enzyme secretion, and gall bladder contraction. They are also implicated in behavioral processes, including anxiety, memory, and drug addiction.etc. However, the development of drugs against cholecystokinin receptors (CCKRs) is challenging mainly due to the lack of precise structural information.
In recent studies published in Nature Chemical Biology, two research teams led by JIANG Yi, WANG Mingwei, H.Eric XU, ZHAO Qiang, WU Beili from Shanghai Institute of Materia Medica of Chinese Academy of Sciences and by ZHAO Suwen from ShanghaiTech University, together revealed mechanisms of ligand recognition, activation and G protein coupling specificity of CCKRs.
The researchers solved three crystal structures of the human CCKAR in complex with two small-molecule antagonists (lintitript and devazepide) and one full agonist NN9056, as well as five cryo-electron microscopy (cryo-EM) structures of CCK-8 activated CCKAR in complex with three G proteins (Gi, Gs, and Gq) and gastrin activated CCKBR coupled to two G proteins (Gi and Gq). Researchers systematically elucidated the mechanisms of CCKRs recognization by ligands, CCKRs activation, and G protein promiscuity of CCKAR, all of which provide fundamental information for drug discovery of CCKRs.
The researchers presented the structures of sulfated CCK-8 bound CCKAR in complex with Gq, Gs, and Gi heterotrimers at global resolutions of 2.9 angstrom, 3.1 angstrom, and 3.2 angstrom, respectively. This work uncovered the binding mode of endogenous peptide CCK-8. The sulfo-tyrosine in CCK-8 was found to be crucial to high affinity of endogenous peptide for CCKAR.
Consistently, the researchers found that Gq protein showed the most potent coupling activity of CCKAR. These results supported Gq as the predominant transducer of CCKAR and highlighted importance of the interface area in G protein coupling selectivity.
Moreover, the research groups reported three crystal structures of CCKAR bound to small molecular antagonists and a peptide agonist, as well as two cryo-EM structures of Gi- and Gq-coupled CCKBR complexes. Their work revealed the binding mode of CCKRs by both peptide and small molecule ligands and identified pivotal roles in recognizing CCKAR by devazepide and lintitript, thus providing a template for designing drugs targeting CCKRs.
Combining the inactive and active structures of CCKAR with the molecular simulation analysis, the researchers proposed the stepwise activation process of CCKAR.
Together, these findings offered the first insight into ligand recognition and activation of the two CCK receptors and a new opportunity for designing drugs targeting CCKRs.
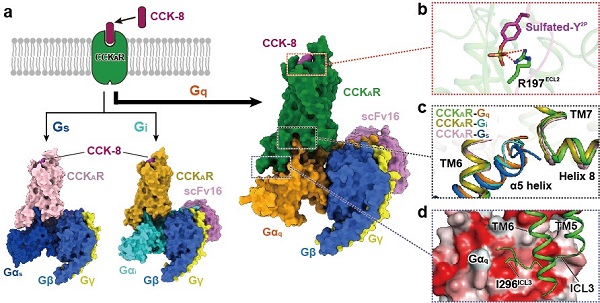
Ligand recognition and G protein coupling promiscuity of CCKAR (image by H.Eric XU’s laboratory)
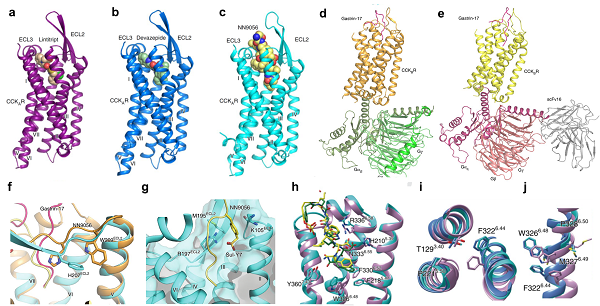
Ligand selectivity and receptor activation mechanism of both CCKAR and CCKBR (image by ZHAO Qiang’s laboratory)
Links:
https://www.nature.com/articles/s41589-021-00841-3
https://www.nature.com/articles/s41589-021-00866-8
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




