Researchers Revealed Mechanism of Autoimmune Hyperthyroidism and Hypothyroidism
Thyroid hormone is one of the most important metabolic hormones whose synthesis is tightly regulated by thyroid stimulating hormone (TSH). TSH activates TSH receptor (TSHR) to stimulate the production of thyroid hormone from thyroid. Graves' disease (GD) is the main disease phenotype of hyperthyroidism. It has been confirmed that TSHR contains an extracellular α subunit which could dissociate from the cell membrane and become an autoantigen to induce various types of antibodies against TSHR, resulting in Graves’ disease and hypothyroidism. In addition to mediating the normal physiological function of the thyroid, TSHR is also involved in the occurrence of thyroid diseases.
However, the structural information on TSH and TSHR is lacking due to the complexity and instability of these proteins. At present, TSH is used for adjuvant treatment of thyroid cancer and is also an important drug target for the treatment of McCune-Albright syndrome, pituitary TSH adenoma, and primary congenital hypothyroidism. The molecular mechanism of how TSH acts on TSHR to trigger thyroid hormone secretion is still unclear. TSHR can trigger autoimmune hyperthyroidism or hypothyroidism, but how human activating antibodies and inhibitory antibodies act on TSHR remain unknown. The molecular mechanism of how small-molecule allosteric agonists activate TSHR is also elusive.
In a study published in Nature, a team of researchers led by H. Eric XU from Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, JIANG Yi from Lingang Laboratory, and ZHANG Shuyang from Peking Union Medical College Hospital, revealed the mechanism of hormone- and antibody-mediated activation of the human TSHR.
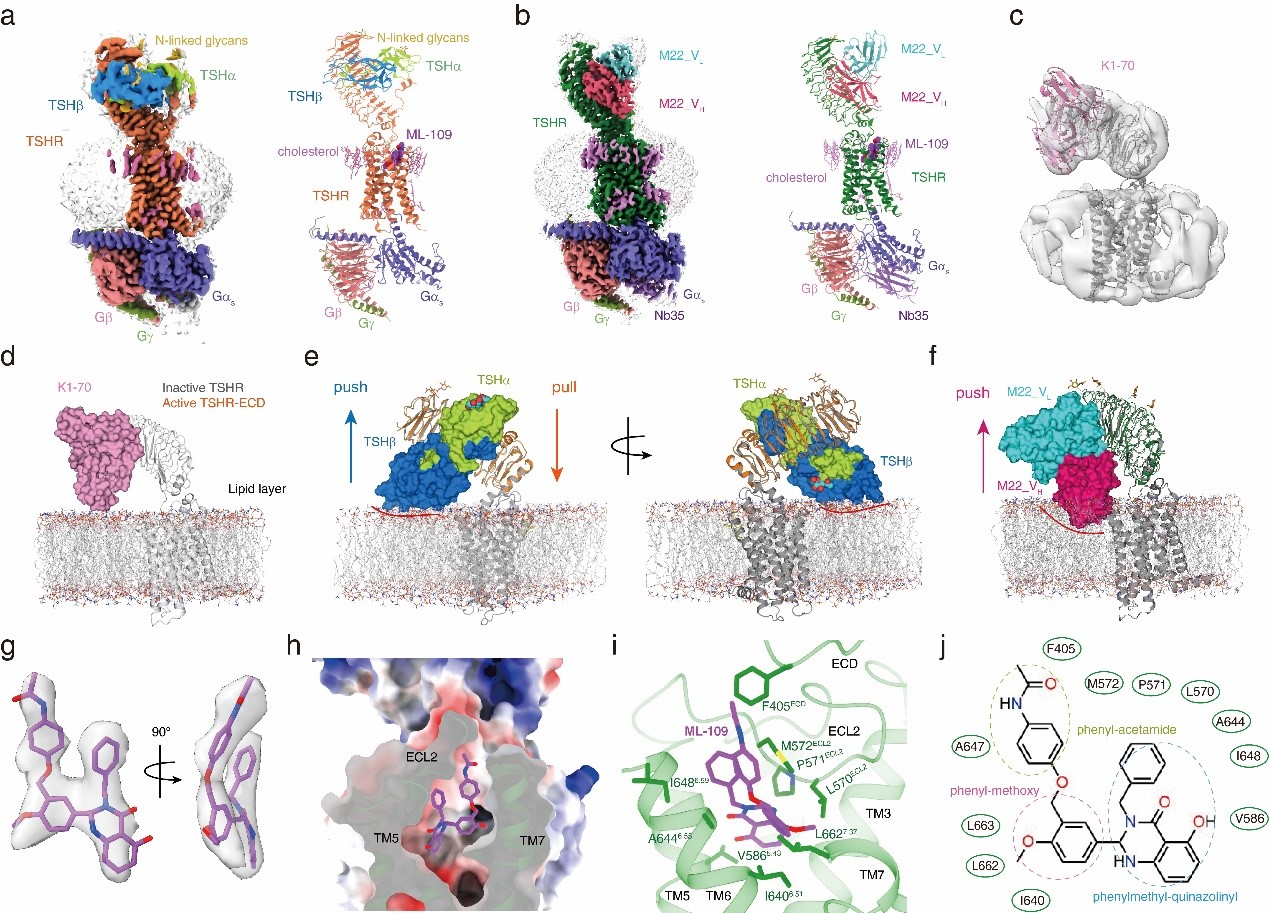
The researchers used single-particle cryo-electron microscopy to solve active structures of TSHR with TSH and an activating autoantibody M22, both bound to an allosteric agonist ML-109, as well as an inactivated TSHR structure with inhibitory antibody K1-70. Further inspection of the structures revealed the key amino acid residues determining the recognition specificity of TSH by TSHR.
Structural comparison of the active and inactivated TSHR revealed that TSH binding pushes a rotation of the extracellular domain (ECD) from the inactivated position to the upright, active conformation. In the inactivated position, K1-70 does not clash with the membrane layer. In contrast, the superposition of the ECD of the active TSHR to that of the inactivated TSHR revealed that TSH will clash with the membrane layer, thus pushing the receptor ECD upward rotation, called “push”. Meanwhile, the extended hinge loop from the receptor hinge region interacting was observed with TSH, which will further pull the overall receptor ECD upward rotation, that is "pull". Combining the two effects, the researchers confirmed that the activation of TSHR by TSH still follows the "push and pull" activation model.
In addition, the researchers clarified the structural basis of the inactivating antibody inhibits TSHR activation by blocking TSH binding. Superposition of the TSHR ECD from the M22 bound structure with the inactivated ECD structure revealed that the heavy chain of M22 will clash with the membrane layer, consistent with the push model of the TSHR activation. However, interactions between M22 and the hinge region were not observed, thus suggesting that antibody-mediated TSHR activation is not mediated through a pull mechanism, as seen in TSHR activated by TSH.
The researchers also analyzed the detailed interactions between the small molecule allosteric agonist ML-109 and TSHR. They identified the key residues that determined the specificity of ML-109 for TSHR, combining with mutagenesis.
This study provides new insights and structural basis for antibody or small molecules drug discovery targeting TSHR therapeutically important receptor.