Aberrant Mitochondrial ClpP Activation Suppresses Pancreatic Ductal Adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is a fatal disease with an extremely poor prognosis, clearly highlighting the need for novel therapies. Mitochondria supply energy for maintaining cellular biochemical reactions such as oxidative phosphorylation (OXPHOS) and tricarboxylic acid cycle. At present, inhibition of respiratory chain complexes to intervene OXPHOS function is a promising antitumor strategy. Human caseinolytic protease P (ClpP) is a highly conserved ATP-dependent serine protease located in the mitochondrial matrix. Dysfunction of ClpP can impair the respiratory chain complex homeostasis, thus inhibiting OXPHOS function and causing cell death. Targeted intervention of ClpP is a potential strategy for antitumor research, but whether small-molecule agonists can inhibit pancreatic cancer still remains unclear.
In a recent study published in Cell Chemical Biology, a team of researchers led by YANG Cai-Guang from Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, has reported a novel and active ClpP activator, and conceptually verifies that aberrant ClpP activation is a potential strategy to inhibit PDAC proliferation.
The researchers examined the feasibility of targeting ClpP as a treatment for PDAC. The studies began by noting a correlation between CLpP expression levels and survival rates in PDAC patients, and observed a reduction in cell proliferation when ClpP is overexpressed in vitro and in vivo. The researchers then screened a compound library and designed a novel ClpP activator ZG111, with improved enzymatic activity and binding affinity towards mitochondrial ClpP. The co-crystal structure of ClpP/ZG111 revealed its molecular and mechanistic basis for activation. ZG111 activated ClpP and degraded multiple respiratory chain complex proteins in mitochondria, which impaired OXPHOS function and induces apoptotic cell death. Transcriptome analysis revealed that ClpP activation induced activation of JNK/c-Jun pathway and endoplasmic reticulum stress response in pancreatic cancer cells. Moreover, ZG111 inhibited both PDAC cell-derived and patient-derived xenografts with different KRAS genetic backgrounds in mice models.
This study provided a primary proof-of-concept that targeted mitochondrial proteome homeostasis may be a new option for pancreatic cancer therapy. The finding that ClpP is an effective target in pancreatic cancer may help address an unmet clinical need. More generally, this study reported a new skeletal ClpP activator and provided a new insight into understanding the structures and functions of ClpP.
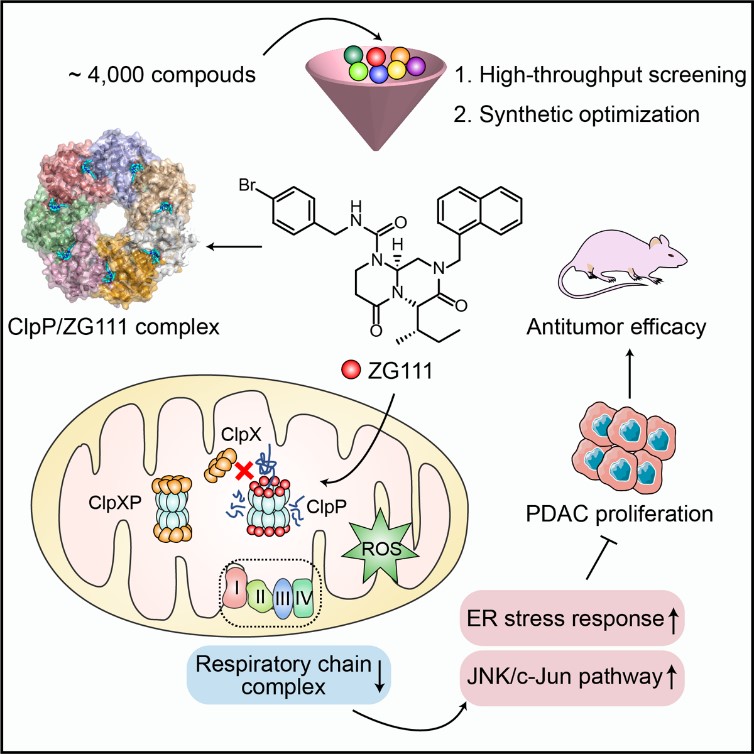
ClpP activator disrupts mitochondrial function against pancreatic cancer (Image by YANG Cai-Guang's laboratory at SIMM)
DOI: https://doi.org/10.1016/j.chembiol.2022.07.002
Link: https://www.sciencedirect.com/science/article/abs/pii/S2451945622002458
CONTACT:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




