Researchers Reveal the Ligand-Recognition Mode of Adenosine Receptor A2BR
Adenosine (ADO) is ubiquitously distributed in human tissue and organ and regulates a multitude of physiological and pathological processes. It is an endogenous ligand that shows relatively low affinity (in micromolar ranges) to A2BR compared with other adenosine receptor subtypes (nanomolar affinity). ADO/A2BR signaling plays a tissue-protective role in acute disease models, such as myocardial ischemia and acute lung injury, etc. BAY 60-6583, a potent and selective A2BR agonist, has cardioprotective effects and increases the secretion of cytokine in the CAR-T cells to eliminate tumor cells. These findings developed A2BR as a potential drug target for the treatment of myocardial ischemia, aging, obesity, cancer, et.al.
In a study published Cell Discovery on 28th December, 2022, A team of researchers led by H.Eric Xu (XU Huaqiang) and XIE Xin from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, in collaboration with a group led by JIANG Yi from Lingang Laboratory, revealed the ligand binding and selectivity of human adenosine receptor A2BR from structural views.
The structures demonstrated a conserved adenosine-binding mode and provide a template for understanding the low adenosine activity against A2BR. They also explained the recognition of the nonnucleoside agonist and the high preference of BAY 60-6583 against A2BR.
In this study, they reported two cryo-electron microscopy (cryo-EM) structures of signaling proteins (Gs) coupled A2BR bound to its endogenous ligand ADO and a non-nucleoside selective agonist BAY 60-6583. The endogenous agonist ADO binds to the orthosteric binding pocket of A2BR, primarily through hydrogen bonds and hydrophobic interactions. It contained a highly conserved mode across ARs. Furthermore, they explored the ligand root-mean-square deviation (RMSD) by molecular dynamics simulations to evaluate the binding stability of ADO in ARs. A2BR showed a relative instability in the binding site and tends to drift out of it, which may explain its weaker binding affinity. In addition, the deeper insertion of BAY 60-6583 relative to ADO results in additional hydrophilic interactions with A2BR pocket residues. They further identified the valine at position 6.51 as a determinant for the high selectivity of BAY 60-6583 for A2BR.
This study provided insights into the ligand-binding, subtype selectivity, and activation for A2BR and a basis for the designing of subtype-specific ligands targeting adenosine receptors.
Link: https://www.nature.com/articles/s41421-022-00503-1
DOI: https://doi.org/10.1038/s41421-022-00503-1
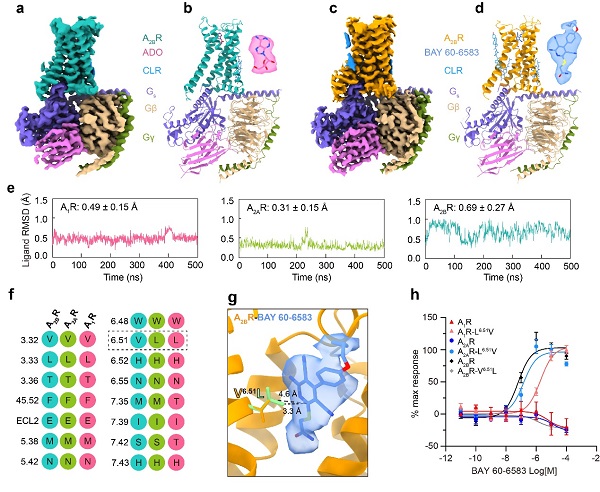
Caption: The cryo-EM structures of adenosine receptor A2BR bound to ligands
Image by XU Huaqiang Eric's laboratory at SIMM
CONTACT:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




