Researchers Discover YTHDF2 Inhibition Potentiate Radiotherapy Anti-Tumor Efficacy
Radiation therapy (RT) is employed in 50-60% of cancer patients. Despite continuous technological and therapeutic improvements, the majority of patients experience treatment failure either locally (due to tumor radioresistance) or at distant metastatic sites. Preclinical data indicates an immune stimulatory effect of ionizing radiation (IR) alone or in combination with checkpoint blockade. Encouraged by these promising preclinical results, an increasing number of clinical trials combining checkpoint blockade with IR have been launched. However, the treatment had limited effectiveness and a consistent meaningful interaction between checkpoint immunotherapy and radiotherapy in humans remains to be established. RNA N6-methyladenosine (m6A) modification is implicated in cancer progression. YTHDF1/2/3 and YTHDC1/2 recognize m6A-modified RNA to regulate RNA metabolisms. Recent studies have suggested m6A readers are implicated in tumor growth in various cancer types. However, only a few studies have reached the impact of reader proteins on antitumor immune response especially in relation to radiotherapy and immunotherapy has not been explored in depth.
In a study published in Cancer Cell on May 26, a team of researchers led by LUO Cheng from Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, in collaboration with groups led by CHUAN He, Ralph R Weichselbaum and Hua Laura Liang from University of Chicago, proposed that YTHDF2 was a promising target to improve radiotherapy and RT/immunotherapy combinations on the basis of previous cooperative study (Nature Chem 2015).
Researchers found that ionizing radiation (IR) induced immunosuppressive myeloid-derived-suppressor-cell (MDSC) expansion and YTHDF2 expression in both murine models and humans. Following IR, loss of Ythdf2 in myeloid cells improved antitumor immunity and overcame tumor radioresistance by altering MDSC differentiation, and inhibiting MDSC infiltration and suppressive function. IR-induced YTHDF2 expression relied on NF-κB signaling; YTHDF2 in turn led to NF-κB activation by directly binding and degrading transcripts encoding negative regulators of NF-κB signaling, resulting in an IR-YTHDF2-NF-κB circuit.
To demonstrate that enhanced efficacy of RT by YTHDF2 deficiency can be translated into a clinically relevant strategy, the researchers conducted high-throughput screening and found a small molecule, DC-Y13, as an inhibitor of YTHDF2 and then designed and synthesized DC-Y13-27, a derivative of DC-Y13, to improve its inhibitory activity. DC-Y13-27 could enhance the antitumor effects of radiotherapy and radio-immunotherapy combinations in a manner similar to the deletion of YTHDF2.
Together, these findings demonstrate a mechanism of YTHDF2 function in radiotherapy and provide new insights of YTHDF2 as the potential target to improve radiotherapy and RT/immunotherapy combinations. The YTHDF2 small molecule inhibitor can not only be a probe tool for the functional study of YTHDF2, but also promote the drug development of YTHDF2 and its application in clinical research.
LINK:https://www.cell.com/cancer-cell/fulltext/S1535-6108(23)00163-0
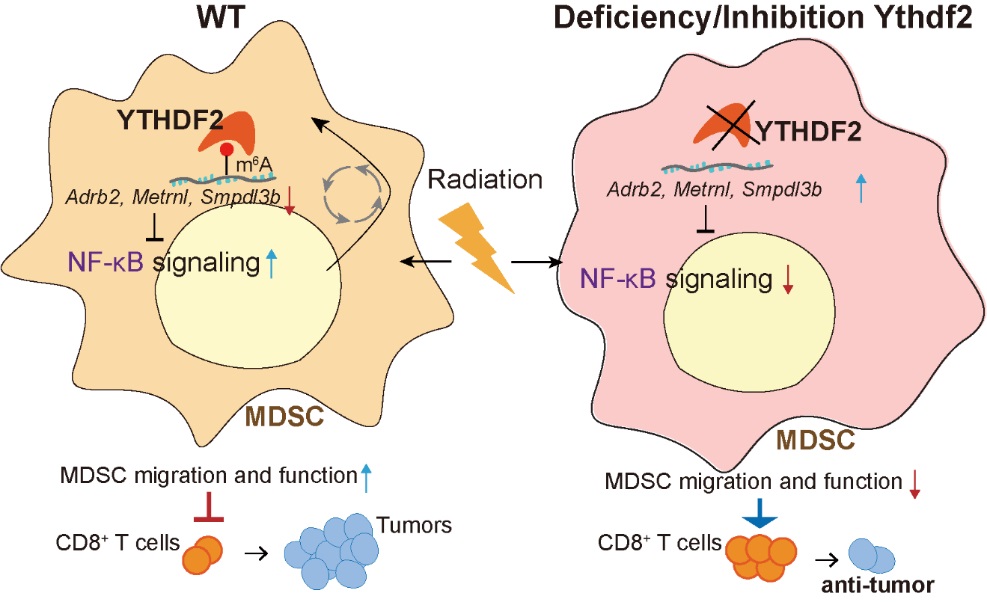
schematic depicting the molecular mechanism through which YTHDF2 inhibition potentiates anti-tumor efficacy of radiotherapy (Image by University of Chicago, 2023)
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




