Chinese Scientists Discover Conserved and Druggable Pocket for Small Molecule Agonists in Class B GPCRs
G protein-coupled receptors (GPCRs) are the largest family of cell surface receptors, regulating various life activities in the human body and closely related to diseases. Class B GPCRs are an important type of peptide hormone receptor, including 15 hormone receptors such as glucagon receptor (GCGR), glucagon-like peptide-1 receptor (GLP-1R), glucose-dependent insulinotropic polypeptide receptor (GIPR), and parathyroid hormone receptor 1 (PTH1R). Class B GPCRs have emerged as important drug targets for treating obesity, diabetes, osteoporosis, depression, cardiovascular diseases, cancer and other diseases. However, drug development targeting these receptors, especially small molecule agonist drugs, has been challenging.
In a study published in Nature, teams of researchers led by H. Eric XU (XU Huaqiang) and ZHAO Lihua from the Shanghai Institute of Materia Medica (SIMM), the Chinese Academy of Sciences (CAS), made a breakthrough discovery in the activation mechanisms of class B GPCR small molecule agonists.
The researchers discovered a new conserved small molecule binding pocket in class B GPCRs. As this pocket is highly conserved across class B GPCRs and can activate multiple receptors, its discovery could provide new opportunities for developing selective small molecule agonists targeting this pocket such as diabetes, obesity and osteoporosis.

Figure 1 The structure of the small molecule, receptor, and G protein complex with PCO371-PTH1R-Gs complex structure in the lower right corner, presenting a new type of small molecule binding pocket (Image by H. Eric XU's lab)
Using cryo-EM, the research team obtained a high-resolution structure of PTH1R bound to a small molecule agonist PCO371. Through structural analysis and functional studies, they discovered a conserved small molecule PCO371-like binding pocket in class B GPCRs. This revealed a novel binding mode for PCO371, and showed PCO371 displays biased G protein signaling.
Specifically, PCO371 binds at the intracellular interface between PTH1R and Gs protein, a site completely distinct from ligand binding pockets reported for any GPCRs. As a biased agonist, PCO371 preferentially signals through G proteins rather than arrestins. Such biased agonists can help reduce arrestin-mediated side effects and improve drug safety and efficacy (Figure 2).
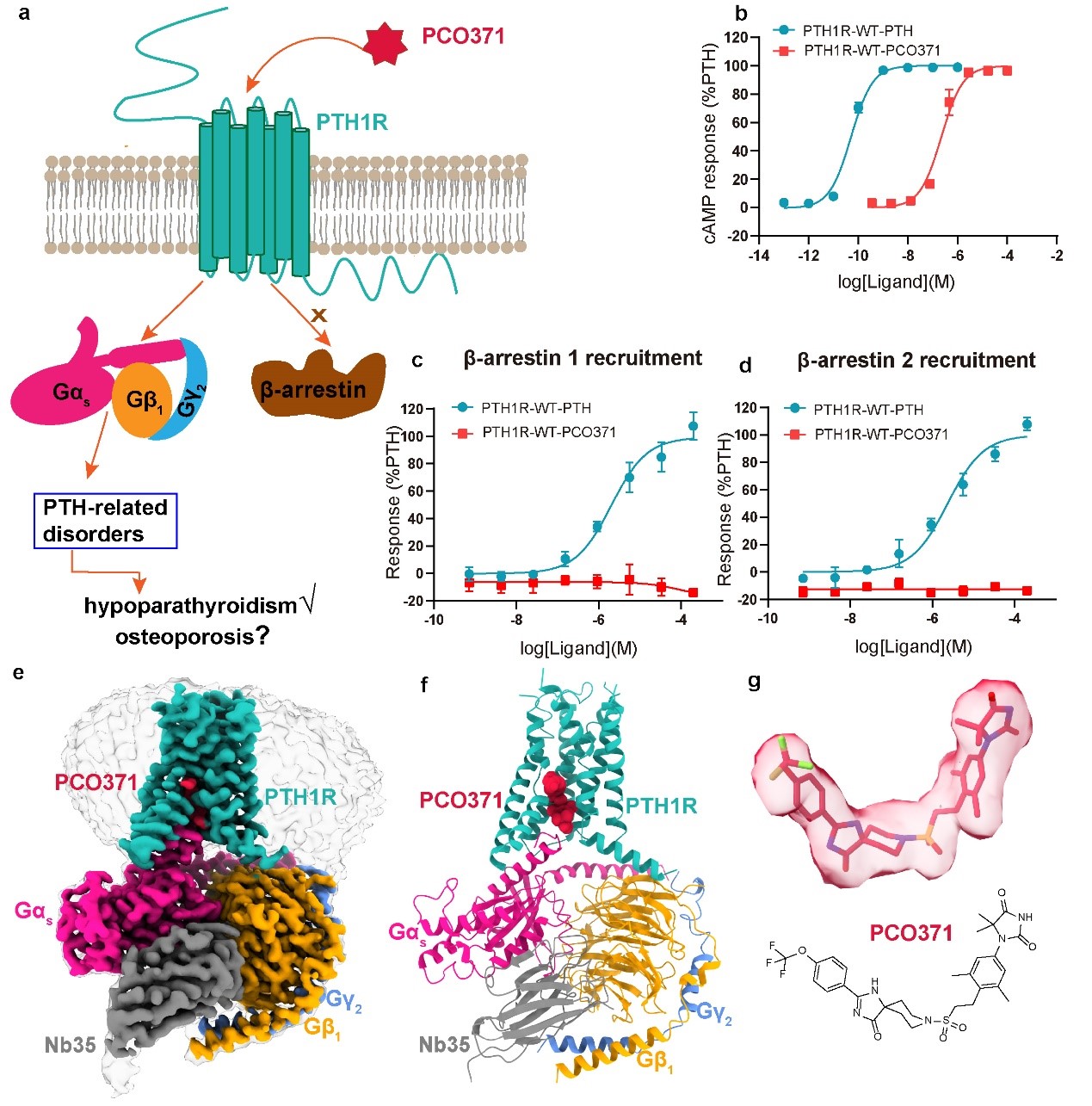
Figure 2 The figure also shows PCO371 preferentially activates the G protein signaling pathway but not the arrestin signaling pathway. The high-resolution structure of the PCO371-PTH1R-Gs complex obtained through cryo-electron microscopy, as well as the new ligand binding pocket presented in the structure (Image by H. Eric XU's lab)
Moreover, sequence analysis revealed key residues comprising the PCO371 pocket are highly conserved across class B GPCRs. PCO371 could activate 8 members of class B GPCRs, and mutations of 1-2 residues conferred responsiveness in other receptors (Figure 3).
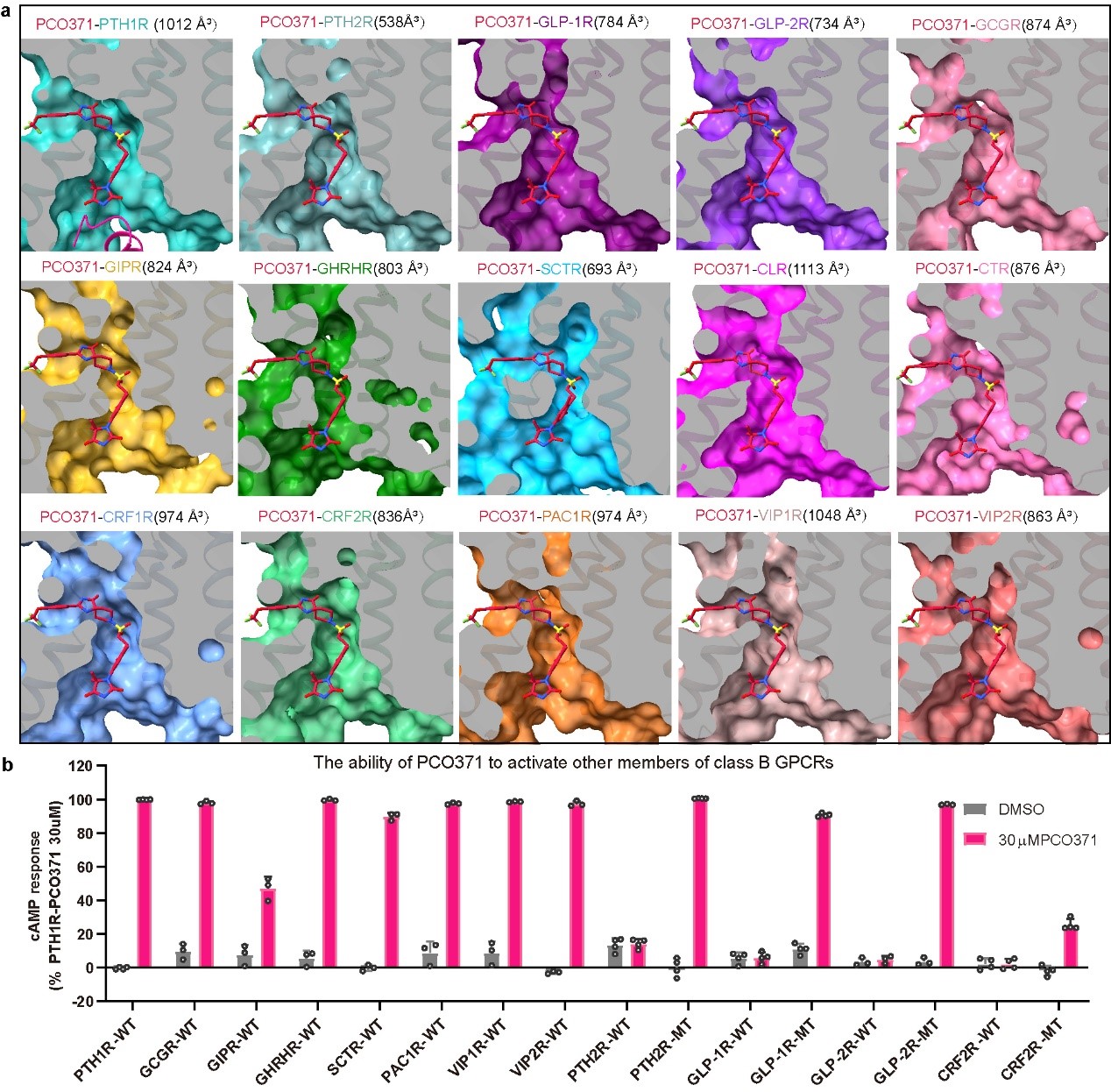
Figure 3 PCO371-like binding pocket in class B GPCRs (Image by H. Eric XU's lab)
These findings elucidate a conserved, druggable pocket for small molecule agonists in class B GPCRs, provide new insights into small molecule drug design targeting class B GPCRs, and open new horizons for developing improved target drugs against diabetes, obesity, osteoporosis and other class B GPCR-involved diseases.
DOI: https://doi.org/10.1038/s41586-023-06467-w
Link: https://www.nature.com/articles/s41586-023-06467-w
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail:diaowentong@simm.ac.cn




