Researchers Uncover Molecular Mechanism of Methamphetamine Binding to Trace Amine Receptor TAAR1
Methamphetamine (METH) abuse is a major health concern, however, there are no FDA-approved treatments. Understanding how METH interacts with its target proteins is crucial for the development of novel medications to address drug addiction. Previous research into the mechanism of METH's effects has mainly focused on the dopamine system, but recent studies suggest it may also directly bind to the trace amine receptor 1 (TAAR1), which plays a key role in psychostimulant abuse-related behaviors. TAAR1 is a receptor in the brain that recognizes various biogenic amines, including the natural compound β-phenethylamine (β-PEA). TAAR1 agonists have been proven effective in treating a range of diseases, including schizophrenia, depression, bipolar disorder, and drug addiction, due to the pivotal role of TAAR1 in modulating monoaminergic systems. Therefore, studying the interactions between METH and TAAR1 through structural biology may aid in addiction treatment and new antipsychotic drug development.
In a study published in Nature on November 7, 2023, a team of researchers led by H. Eric Xu from Shanghai Institute of Materia Medica, Chinese Academy of Sciences, in collaboration with XU Fei from iHuman Institute at ShanghaiTech University, WANG Sheng from Center for Excellence in Molecular Cell Science, and researchers from Shanghai Municipal Bureau of Justice, uncovered the molecular mechanism of methamphetamine binding to trace amine receptor TAAR1.
METH, or "crystal meth", was once used for medical purposes but is now widely abused. It's important to ensure its safe and controlled use, similar to other powerful drugs such as morphine and fentanyl, which have their own clinical applications but carry risks of abuse and addiction. Prof. H. Eric Xu's team has conducted thorough scientific research on critical issues associated with drug addiction. Two publications were featured in Cell (2022, 2023), systematically explaining the mechanisms that occur between opioid receptors and various small-molecule analgesics and endogenous opioids. Their research has established a solid pharmacological basis for the field.
In this study, the researchers used cryo-electron microscopy (cryo-EM) to determine high-resolution structures of the human TAAR1-Gs protein complex simulated by METH, β-PEA, the selective agonist RO5256390, and the clinical candidate SEP-363856. Structural analysis revealed that METH binds to TAAR1 mainly through polar interactions with Asp103 and Tyr294. A hydrogen bond network around the binding site stabilizes METH-TAAR1 interactions. In addition, the extracellular loop 2 (ECL2) of TAAR1 forms a unique "lid" that interacts with the ligands, using Phe186 and other hydrophobic residues. Compared to β-PEA, METH forms weaker polar interactions with Asp103 and Ser107, which explains why β-PEA has a higher binding affinity to TAAR1.
The study also explored the structural pharmacology of clinical candidate SEP-363856 (a dual 5HT1AR and TAAR1 agonist) and selective TAAR1 agonist RO5256390 bound to TAAR1. Structural analysis in combination with mutagenesis experiments revealed how these compounds interact with the receptor and why they exhibit different selectivity and affinity. For example, the common interactions that govern the recognition of SEP-363856 by TAAR1 or 5-HT1AR may provide the structural foundation for the polypharmacology of SEP-363856. RO5256390, on the other hand, has additional interactions with TAAR1, providing selectivity over 5-HT1A due to steric hindrance.
In summary, this study fills a critical gap by providing the long-awaited structures of monoaminergic receptor targeting by METH and lays a strong foundation for the development of new addiction treatments and drugs for psychiatric disorders. In addition, the structural elements governing TAAR1 recognition by METH and other amines elucidated in this study will be essential for pharmacological studies and the development of next-generation drugs.
DOI: 10.1038/s41586-023-06775-1
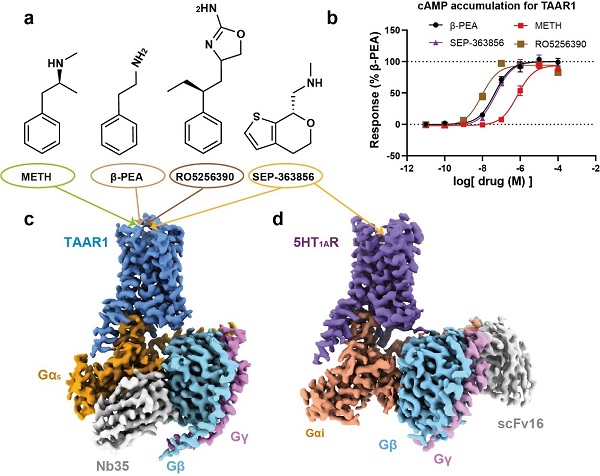
three-dimensional structures of TAAR1 and 5HT1A receptors (Image by H. Eric Xu’s lab, simm)
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




