Innovative Drug Gumarontinib has been Successfully Approved in Japan
On June 24, 2024, Ministry of Health, Labor and Welfare of Japan approved New Drug Application (NDA) of Gumarontinib (SCC244) for the treatment of locally advanced or recurrent non-small cell lung cancer (NSCLC) with MET exon 14 (METex14) skipping mutation.
The drug was developed by a research team led by SHEN Jingkang and GENG Meiyu, in collaboration with DING Jian and GAN Yong from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences. It was further developed by SIMM and Haihe Biopharma Co., Ltd. (referred as “Haihe”), which holds global independent intellectual property rights.
Gumarontinib is an oral, potent and highly selective small molecule MET inhibitor. It has shown excellent pharmacokinetic characteristics, highly effective, durable effectiveness, and a favorable safety profile in NSCLC patients with MET alterations. Gumarontinib has long half-life to achieve sustained target inhibition, low DDI potential to enable less co-medication restrictions, convenient QD regimen.
The indication application is based on data from the successful SCC244-108 (GLORY study, NCT04270591), a pivotal Phase II study evaluating the efficacy and safety of Gumarontinib in locally advanced or metastatic NSCLC patients with METex14 skipping mutations. This indication was approved by NMPA in China on March 7, 2023. The global leading principal investigator is Prof. SHUN Lu from Shanghai Chest Hospital.
A total of 79 patients with MET exon 14 skipping mutations confirmed by the central laboratory were enrolled in the GLORY study, with data cut-off on April 28, 2022. The confirmed overall objective response rate (ORR) per Blinded Independent Review Committee (IRC) was 65.8%, including 70.5% ORR for treatment-naïve patients and 60.0% ORR for previously-treated patients at 12-months follow-up. The median progression-free survival (PFS) was 8.5 months, 11.7 months and 7.6 months for overall population, treatment-naïve and previously treated patients respectively. Additionally, the median overall survival (OS) was 17.3 months and 16.2 months for overall population and previously-treated patients respectively, while it has not reached in treatment-naïve patients to the date of this report.
“The approval of Gumarontinib in Japan will provide a new treatment option for the Japanese NSCLC patients. It also confirms that the GLORY study of Gumarontinib, led by Chinese and Japanese researchers, has gained international recognition and affirmation. It is a new milestone in the journey of China’s bio-innovative drugs onto the global stage. We look forward to seeing more innovative drugs from China, such as Gumarontinib, go global to meet the unmet medical needs of patients.” commented SHUN.
As a national strategic scientific force in innovative drug development, SIMM currently has 55 candidate drugs in clinical research, including 25 in Phase II-III clinical trials and 15 undergoing international clinical trials. So far, SIMM has established a favorable development trend in innovative drug research and development. Through the marketing of Gumarontinib in Japan, SIMM will further refine its scientific objectives, continuously developing innovative drugs with international influence to improve people's health.
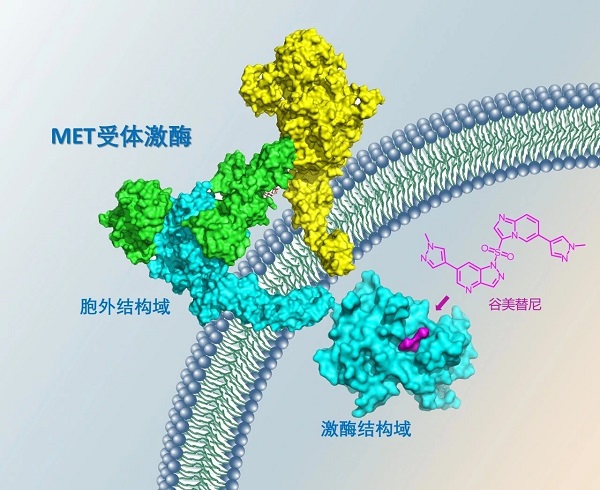
Diagram of the Binding Interaction between Gumarontinib and MET (Image by SIMM)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




