Direct Cytosolic Delivery of siRNA via Cell Membrane Fusion Using Cholesterol-Enriched Exosomes
RNA interference (RNAi) technology has gradually become a cutting-edge technology for treating major diseases, such as genetic disorders and cancer, due to its huge potential in gene expression regulation. However, the efficient delivery and safety of siRNA remain key challenges for its clinical application. Although RNA delivery systems, such as N-acetylgalactosamine (GalNAc) and lipid nanoparticles (LNP), have been explored and used, their efficiency in lysosomal escape is still low (1-4%), limiting their overall efficacy.
Exosomes, as natural nanoscale extracellular vesicles, have gradually become a research hotspot for optimizing siRNA delivery due to their excellent biocompatibility, stability, long-lasting in vivo circulation time, and precise tissue targeting ability. However, traditional exosome delivery mainly relies on endocytosis, which leads to rapid degradation of RNA molecules in lysosomes, thereby limiting delivery efficiency.
In a study published in Nature Nanotechnology, a research team led by GAN Yong and YU Miaorong from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, in collaboration with HU Guoqing’ group from Zhejiang University, combined theoretical modeling with experimental studies to reveal the key role of cholesterol in regulating delivery of RNA drugs by exosomes and the underlying mechanism.
The research team used a controllable membrane engineering strategy to prepare exosomes with different cholesterol contents (such as milk exosomes, ginger exosomes, tumor cell-derived exosomes, etc.). Using technologies like transmission electron microscopy, they comprehensively characterized the cellular uptake behavior of these exosomes. The results showed that increasing of cholesterol content in the exosome membrane significantly enhanced its interaction with the target cell membrane, promoting exosomes entry into the cell through membrane fusion rather than endocytosis, thus bypassing the limitation of lysosomal degradation. Molecular model also revealed that cholesterol-rich exosomes exhibit stronger membrane deformability and can expand their contact area with the cell membrane, thereby delivering siRNA directly to the cytoplasm with high efficiency through membrane fusion mechanism.
In the in vitro experiment, milk exosomes enriched in 30% cholesterol (30%Chol/MEs) successfully delivered PLK1 siRNA, significantly down-regulated PLK1 mRNA and protein expression levels, and induced apoptosis of tumor cells. The effect was significantly better than that of traditional transfection agents Lipo 2000 and RNAiMAX. The researchers further confirmed that 30% Chol/MEs/siPLK1 could effectively inhibit tumor growth in the mouse colorectal tumor model through both oral and intravenous injection, indicating that these special exosomes have great potential as gene therapy vectors.
DOI: 10.1038/s41565-024-01785-0
Link:https://www.nature.com/articles/s41565-024-01785-0
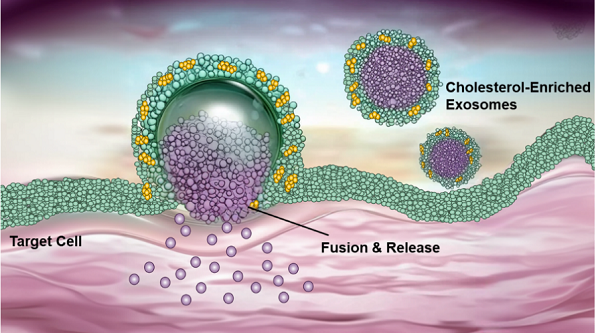
Cholesterol-enriched exosomes merged with the cell surface. (Image by GAN’s lab)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




