Novel Nanovesicle May Improve Immunochemotherapy for TNBC
Interleukin 15 (IL15) is a key cytokine for promoting survival and proliferation of natural killer (NK) cells and CD8+ T cells. However, IL15 based supplementary therapies face challenges such as systemic inflammation and nonspecific stimulation of cancer cells. In addition, triggering tumor cell immunogenic death (ICD) while reducing damage to intratumoral immune cells remains a critical challenge.
In a study published in Advanced Science on December 4, a research team led by Professor LI Yaping from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, and Professor ZHANG Pengcheng from ShanghaiTech University, built a nanovesicle termed DoxFILN. This nanovesicle comprises a membrane presenting IL15/IL15 receptor α complexes (IL15c) and a core of doxorubicin-loaded ferritin (Dox-Fn), enabling bicellular targeting for treatment of triple negative breast cancer.
The researchers conceived that drug delivery strategies targeting different intratumoral cells were critical for modulating multiple cell types within the tumor, ultimately enhancing cancer immunotherapy. Tissue distribution experiments revealed that DoxFILN accumulated more in tumor tissues after intravenous injection. Fluorescence staining revealed that membrane-bound IL15c co-localized with NK cells and CD8+ T cells, while Dox-Fn was predominantly taken up by tumor cells. Flow cytometry demonstrated proliferation and activation of T cells and NK cells. Significant tumor growth inhibition and prolonged survival were observed in 4T1 or EMT6 tumor-bearing mice.
This study represented a significant advancement in cancer immunotherapy. By overcoming challenges associated with traditional IL-15 supplementation and chemotherapy, the new DoxFILN system provided a more targeted, effective, and less harmful approach to cancer treatment. The ability to simultaneously induce ICD in tumor cells while boosting immune response through IL-15c presented a promising strategy for enhancing natural defense against cancer. This dual-action platform could lead to more effective therapies with fewer side effects, potentially offering new hope for cancer treatment.
DOI: 10.1002/advs.202409194
Link: https://doi.org/10.1002/advs.202409194
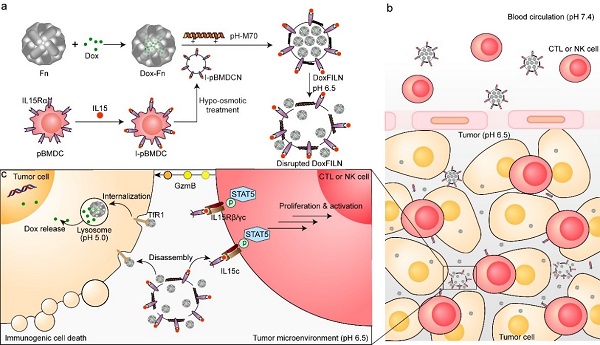
The anticancer effect of DoxFILN on TNBC. (Image by ZHAI Yihui)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




