3D Channel Maze, New Mechanism to Control Drug Release from Multiple Unit Tablets
Controlled drug release systems have been a focus to provide predictable drug delivery. These systems involve various mechanisms, including diffusion of water and drugs, matrix erosion, polymer swelling, osmotic effects, and ion exchange. Among these, diffusion is the fundamental mechanism for most dosage forms. Multi-unit pellet systems, such as multiple unit tablets, represent a novel type of controlled release system, offering advantages like avoiding excessive local drug concentrations, improving high patient compliance, and reducing side effects. Exploring drug release process and mechanism from the perspective of three-dimensional (3D) formulation structures is essential for understanding dosage forms, particularly the in vitro and in vivo behavior of advanced formulations like multiple unit tablets made of pellets.
In a study published in Journal of Controlled Release on December 8, a research team led by ZHANG Jiwen and WU Li from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, along with collaborators, using synchrotron radiation X-ray micro-computed tomography (SR-μCT), revealed “3D channel maze” mechanism to control drug release from multiple unit tablets.
In this study, SR-μCT was applied to obtain 3D images of theophylline multiple unit tablets made of pellets. The overall structure of tablets and internal structural changes of individual pellets during the drug release process were investigated. By combining release kinetics of the entire tablet and individual pellets, a “3D channel maze” mechanism for controlled release was proposed.
Analysis of the internal structure of theophylline multiple unit tablets revealed that the pellets were randomly distributed radially, with a higher number of pellets located on the back side of the tablet compared to the front. Both radial and axial slices indicated that the tablets were primarily composed of three regions: the theophylline pellets, the protective cushion layer, and the matrix layer. Using Reconstructed 3D images, the structure of individual pellets within the tablet were extracted. Each pellet consisted of a core and a coating layer, with diameters ranging from 0.5 to 1.2 mm. The coating layer was relatively uniform and compact, with an average thickness of approximately 100 μm.
The matrix layer and dominant region (including the protective cushion layer and theophylline pellets) played key roles in the release profiles.
During the rapid release stage (initial dissolution phase), the outer matrix layer of the tablet, comprised of drugs and soluble excipients, dissolved rapidly, while the dominant region remained largely intact. Pellets located at the tablet edges, unprotected by the buffer layer, began to dissolve.
In the controlled release stage, as pellets dissolved, small outlets and channels emerged to release dissolved drug molecules. The partially soluble protective cushion layer acted as a barrier, slowing drug release through tortuous pore channels. Over time, these pores and channels between the pellets became interconnected, forming a complex and labyrinthine “3D channel maze” structure. Drug molecules dissolved in the liquid medium had to navigate their way out of this maze. While some molecules exited quickly, others required more time to traverse the maze. The release of drug within the “3D channel maze” was governed by multiple processes, including diffusion of water, dissolution of drug, diffusion within the 3D channel network, and the eventual escape from the maze. The gating effect of the maze played a critical role in achieving controlled release.
The “3D channel maze” mechanism offered a new perspective on controlled release process of theophylline multiple unit tablets. Unlike diffusion-based mechanisms of conventional theories, the “3D channel maze” model emphasized the gating effect of the internal pore network within the tablet. While the drug dissolved and diffused freely within the pore network, its movement was restricted by intricate and tortuous channel structure to elongate the drug release profiles.
DOI: 10.1016/j.jconrel.2024.12.014
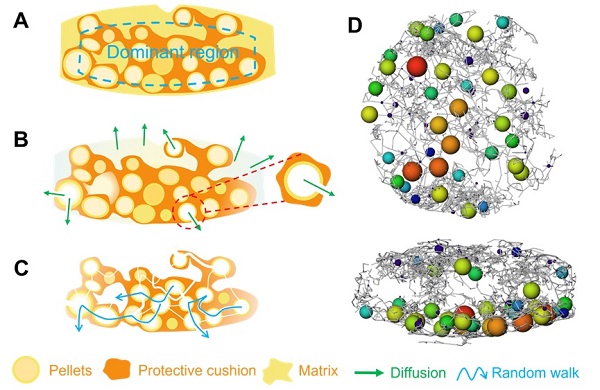
“3D channel maze” mechanism. (Image by ZHANG’s lab )
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




