Study Decodes Mechanisms of Coral-derived Biflorane Terpene Synthase
Bifloranes, also known as serrulatanes, feature a distinct 6,6-bicyclic framework and a prenyl side chain, showcasing a wide range of biological activities such as anti-inflammatory, antimalarial, and antitumor effects. These compounds are commonly found in marine corals, sponges, and plants. Despite recent studies identifying a few biflorane terpene synthases, the catalytic mechanism and structural basis for the formation of marine biflorane scaffolds remain largely unknown. Additionally, only one crystal structure of coral terpene synthase has been obtained so far, limiting our understanding of marine coral terpenoid biosynthesis.
In a study published in Science Advances on February 26, a research team led by XU Baofu from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences (CAS), along with GUO Yuewei from the SIMM, WU Ruibo from the Sun Yat-sen University, and WANG Chengyuan from the Shanghai Institute of Immunity and Infection of CAS, using genome mining and enzymology techniques, discovered several coral terpene synthases and thoroughly elucidated the catalytic mechanism of a biflorane synthase, PcTS1.
The research focused on the genomic analysis of the sea whip, Paramuricea clavate, leading to the discovery of six terpene synthases (TSs), including a biflorane synthase, PcTS1. This discovery enabled detailed mechanistic studies of marine biflorane scaffold formation. Further experiments using deuterium and fluorine labeling clarified the cyclization pathway of PcTS1. During this process, GGPP isomerizes to form GLPP, introducing a 2Z double bond, followed by a 1,10-cyclization and a 1,3-hydride shift, resulting in a 1,6-ring closure. The pathway continues with two additional 1,2-hydride shifts, ultimately culminating in deprotonation. This catalytic route disproved the previous hypothesis that suggested two 1,3-hydride shifts occurred during the process.
Through crystallization experiments, the research team determined the crystal structure of PcTS1. This discovery not only deepened the understanding of the biflorane synthase PcTS1 but also facilitated further quantum mechanics/molecular mechanics (QM/MM)-calculations. These calculations confirmed the plausibility of a cyclopropane 2,6-cyclization and opening, occurring between two 1,2-hydride shifts before the final deprotonation. Additionally, the calculations significantly clarified the role of pyrophosphate as the base responsible for deprotonation, a challenging aspect in the initial mechanism proposal that was difficult to confirm through labeling experiments.
Further mutational studies identified a ten-membered intermediate shunt product from the mutant PcTS1I254A, supporting the cyclization mechanism that begins with 1,10 cyclization in PcTS1. Subsequent mutagenesis experiments resulted in the creation of various unique terpene scaffolds, aligning with the carbon cation intermediates proposed from isotopic labeling and QM/MM studies. Notably, this included a cage terpene structure produced by the PcTS1S338I variant, and two distinct cyclopropane-containing terpene frameworks from the PcTS1I59L and PcTS1F221V mutants. This mechanism-directed engineering of PcTS1 expanded the diversity of terpene scaffolds and enhanced the potential biocatalysts available for terpene synthesis.
These findings not only enhanced the understanding of coral biforane formation mechanisms but also marked the first domestic report on marine coral biosynthesis in China, significantly boosting local research in this field. Additionally, the study offered a solution to the long-standing challenge of sourcing bioactive compounds from marine corals by utilizing synthetic biology approaches for efficient heterologous production.
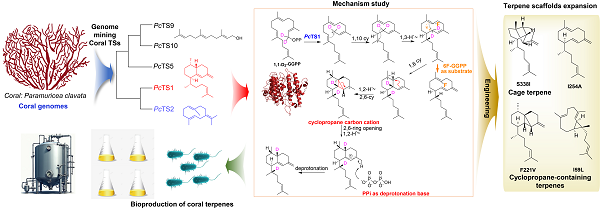
Elucidating terpenoid biosynthesis through genome mining and mechanistic studies can facilitate the efficient heterologous production of bioactive marine coral terpenoids. (Image by XU Baofu)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




