Researchers Discover Covalent ALKBH5 Inhibitor with Novel Mechanism Against Leukemia
As an emerging field of RNA epigenetics in molecular biology, m6A modification has been shown to affect transcription and expression of key pathogenic gene sets in various diseases. ALKBH5, an eraser protein of m6A modification, can affect disease progression by regulating m6A modification in RNA. In acute myeloid leukemia, ALKBH5 overexpression enhances stability of downstream oncogene transcriptomes, leading to overexpression of oncoproteins and ultimately causing malignant progression of cancer. However, regulating ALKBH5 activity through chemical small molecule intervention remains a significant challenge.
In a study published in Angewandte Chemie International Edition on March 2, which was selected as a "Very Important Paper", a research team led by Prof. LIU Hong and Prof. YANG Cai-guang from the Shanghai Institute of Materia Medica of Chinese Academy of Sciences, identified compound 18l as a covalent inhibitor targeting non-catalytic Cys200 residue of ALKBH5. The team verified its Cys200 covalent mechanism, in vitro and in vivo activity, most importantly, demonstrated intracellular targeting of ALKBH5.
Cys200 residue is a non-catalytic covalent site unique to ALKBH5, located near single-stranded RNA (ssRNA) substrate binding pocket of ALKBH5. The researchers envisioned that targeting this site for covalent modification could interfere with substrate binding to ALKBH5 and achieve selective activity inhibition. Through preliminary screening and structure-activity relationship studies, the researchers obtained compound 18l (ALKBH5 IC50 = 620 nM) with maleimide as a covalent warhead.
A variety of in vitro experiments confirmed that 18l inhibits ALKBH5 enzymatic activity by covalently modifying Cys200, thereby preventing substrate binding to ALKBH5. In addition, 18l exhibited good selectivity for ALKBH5 over FTO and various Cys proteases. At the cellular level, compound 18l was able to inhibit AML cell proliferation (NB4 cell IC50 = 630 nM), promote AML cell apoptosis, induce differentiation into normal cells, block cell cycle, and inhibit ALKBH5-mediated signaling pathways. In addition, 18l could increase m6A level in RNA in NB4 cells, indicating that the compound could inhibit ALKBH5 in cells.
To verify correlation between 18l's enzyme-level ALKBH5 inhibitory activity and anti-AML cell phenotype, the researchers conducted non-modification targeting verification and ABPP-based chemical proteomics studies. In the non-modification experiment, 18l could change protease resistance and thermal stability of ALKBH5, thereby proving its intracellular targeting engagement. In ABPP proteomics experiment, a chemical probe based on 18l could bind to ALKBH5, further confirming that 18l can bind to ALKBH5 in AML cells. Finally, an in vivo antitumor pharmacodynamic study was conducted in an AML cell mouse xenograft tumor model. At doses of 1 mg/kg and 2.5 mg/kg, 18l effectively inhibited tumor growth, achieving tumor inhibition rates of 66.3% and 76.8%, respectively, without observable adverse effects, demonstrating efficacy and safety in vivo.
In this study, the research team designed covalent drugs targeting Cys200 residue of ALKBH5, thereby interfering with substrates binding to ALKBH5 and exerting an inhibitory effect. Through preliminary screening and structure-activity relationship studies, the preferred compound 18l with a maleimide as a covalent warhead was found. Mass spectrometry verification and mutation experiments confirmed that 18l inhibited ALKBH5 activity by covalently modifying Cys200, further preventing substrate binding. The compound exhibited potent ALKBH5 inhibitory activity and high selectivity, and showed excellent anti-tumor effects in in vitro and in vivo pharmacodynamic studies on AML. Further mechanistic studies clarified that 18l exerts anti-AML effects by modulating RNA epigenetic pathways. These findings provide new guiding ideas for precise chemical regulation in the field of RNA epigenetics.
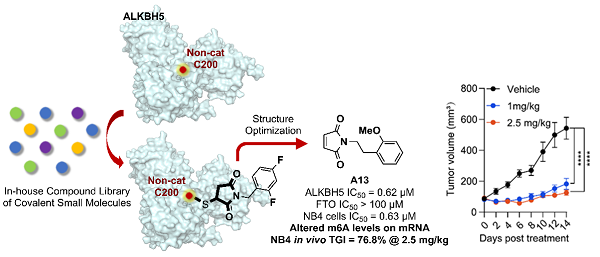
Schematic Diagram of the Discovery of Covalent ALKBH5 Inhibitors for Targeted Leukemia Therapy (Image by LIU’s group)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




