Study Identifies Structural Basis of CysLT2R Activation by LTD4
Cysteinyl leukotrienes (CysLTs) are potent bronchoconstrictors originating from 5-lipoxygenase pathway, playing pivotal roles in inflammatory diseases. They induce airway constriction, excessive mucus production, and vascular leakage. These lipid mediators exert their effects by activating two G protein-coupled receptors, CysLT1R and CysLT2R. Unlike CysLT1R, which predominantly functions in lungs, CysLT2R operates across multiple organ systems. CysLT2R is highly expressed in stomach, small intestine, and rectum, linking it to gastrointestinal inflammation. Additionally, the unique presence of CysLT2R in heart, brain, and adrenal glands suggests its involvement in cardiovascular and neurological disorders. This widespread distribution positions CysLT2R as both a local inflammation amplifier and a systemic disease bridge. However, the activated conformation of CysLT2R receptor upon binding to an endogenous ligand remains elusive, presenting two major challenges. First is the uncertainty in mechanism, as the precise molecular details of "ligand-receptor" coupling cannot be elucidated. Second are drug-discovery hurdles, characterized by drug-screening blind spot and low hit-rate in identifying potential drugs. Together, these issues hamper treatment of CysLT2R-related diseases.
In a study published in PNAS on April 7, a research team led by YIN Wanchao from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, JIANG Yi from the Lingang Laboratory, and ZHANG Shuyang from the Peking Union Medical College, used single-particle cryo-electron microscopy (Cryo-EM) to obtain a high-resolution structure of CysLT2R binding to endogenous ligand cysteinyl leukotriene D4 (LTD4). The obtained structure exhibited an activated conformation with a resolution range of 3.15 Å and revealed that CysLT2R binds to LTD4 to recruit downstream Gαq proteins.
The research team made several remarkable discoveries based on the high-resolution structure. LTD4 can enter the binding pocket through a vertical transverse channel between transmembrane domain helix (TM) 4 and 5. The polar head of LTD4 is anchored securely by a complex hydrogen-bond network with the receptor's second extracellular loop (ECL2) and transmembrane helices. In contrast, the alkyl tail of LTD4 engages in hydrophobic interactions with the receptor, stabilizing the ligand-binding pocket. This "hydrogen-bonding at the top, hydrophobic-locking at the bottom" dual-stabilization mechanism highlights evolutionary conservation in lipid-rich GPCR families. Additionally, the study revealed that TM3 plays a critical role in inducing agonists and triggering receptor activation. When leukotriene D4 binds to CysLT2R, it triggers a conformational change in TM3, transforming the receptor into its active form and unleashing a cascade of downstream signaling events. Interestingly, the study found that in their inactive state, CysLT1R and CysLT2R exhibit almost identical TM3 conformations, suggesting a universal activation "blueprint" for the entire CysLTR family.
This research significantly advances the understanding of how CysLT2R is activated by its endogenous ligand LTD4. The enhanced understanding will facilitate the design of structure-based anti-inflammatory and anti-allergic drugs, potentially improving treatment for diseases associated with leukotriene pathway dysregulation. Cardiovascular disease, psychiatric disorders, and certain cancers could benefit from targeted therapies that block CysLT2R, offering new hope for patients suffering from these debilitating conditions.
Keyword:
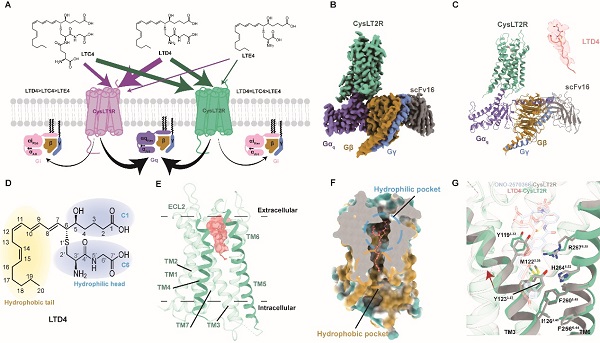
(A)Schematic representation of signal transduction between LTC4, LTD4, LTE4, and CysLT1/2R. (B) Cryo-EM density map of LTD4-CysLT2R-Gαq complex. (C) Structural model determined after refinement in the cryo-EM map and LTD4 density map. (D) LTD4 chemical formula. (E) LTD4 binding pocket of CysLT2R. (F) Sliced surface representation of the LTD4-binding pocket in CysLT2R. (G)Detailed comparison of interactions between LTD4-binding and ONO 2570366 binding (PDB:6RZ6) CysLT2R. (Image by JIANG Mengting)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




