Dynamic Cap-Mediated Substrate Access and Potent Inhibitor Design of Monkeypox Virus I7L Protease
Monkeypox virus (MPXV), a member of the Orthopoxvirus genus long endemic in Africa, triggered a global outbreak in 2022 and was declared a Public Health Emergency of International Concern by the World Health Organization (WHO). The I7L protease, essential for viral maturation, has emerged as a key target for antiviral drug development. However, efforts to develop effective inhibitors have been hampered by limited understanding of substrate recognition, absence of structural data, and lack of reliable enzymatic assays.
In a study published in Advanced Science on April 8, a research team led by Prof. XU Yechun from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences (CAS), Prof. ZHANG Leike from the Wuhan Institute of Virology of CAS, reported the first high-resolution crystal structure of the MPXV I7L protease. Unique conformational changes of this protease were also identified, which enabled the design of nanomolar-level covalent inhibitors as promising leads for broad-spectrum antiviral development.
In this study, three high-resolution crystal structures of the MPXV I7L protease were solved, revealing that the protease forms a stable dimer. In addition, a flexible “cap region” near the active site was identified, exhibiting both open and closed conformations, indicating its role in dynamically regulating substrate access. Structural analysis followed by molecular dynamics (MD) simulations confirmed that this cap functions as a conformational switch during substrate binding.
Using AlphaFold3-based modeling of protease-substrate structures and Quantum Mechanics/Molecular Mechanics (QM/MM) calculations, the researchers explored detailed molecular mechanisms of substrate recognition and hydrolysis by the I7L protease. The key roles of S1–S5 subsites in the substrate binding and a rate-limiting step (deacylation) in the hydrolysis reaction profile were revealed.
Based on these insights, the researchers designed peptidomimetic covalent inhibitors featuring a nitrile warhead. To evaluate inhibitory activity of compounds, a high-throughput Fluorescence Resonance Energy Transfer (FRET)-based assay was developed, which led to the discovery of several potent hits. Among them, compound 11 showed the most potent inhibitory activity against the I7L protease, with an IC₅₀ value of 69 nM. In cell-based assays, it effectively inhibited vaccinia virus replication with an EC₅₀ value of 6.0 μM and showed no significant cytotoxicity at concentrations up to 400 μM (CC₅₀ > 400 μM).
The research team also performed multiple sequence alignment across orthopoxvirus species and monkeypox clades (Clade I, IIa, IIb), revealing that the I7L protease is highly conserved. No mutations were observed at key inhibitor-binding sites, suggesting that these inhibitors could offer broad-spectrum activity against various orthopoxviruses and their variants.
This work provided a comprehensive understanding of the MPXV I7L protease’s structure, dynamics, and function, and presented a successful example of structure-based design of covalent peptidomimetic inhibitors. These findings established a solid foundation and provided valuable lead compounds for the development of antiviral drugs against MPXV and other orthopoxviruses.
Link: https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202501625
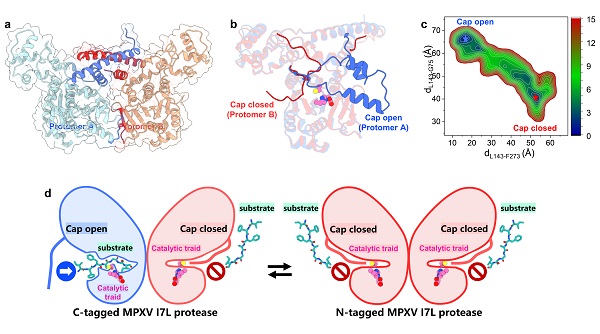
First-in-class inhibitor design based on the first crystal structure of MPXV I7L protease
(Image by XIONG Muya)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




