Self-Assembling Glycopeptide Defeats Superbugs by Disrupting Cell Structures and Virulence Pathways
Antimicrobial resistance (AMR) represents an escalating global health crisis, responsible for an estimated 1.27 million deaths directly caused by resistant infections in 2019 and nearly 5 million deaths associated with AMR. Between 2025 and 2050, projections suggest 39.1 million deaths and 169 million AMR-related fatalities. To address this pressing challenge, scientists are urgently seeking new-generation antibiotics capable of tackling multidrug-resistant (MDR) “superbugs” .
In a study published in Journal of the American Chemical Society (JACS) on April 8, a research team led by GUAN Dongliang from the Shanghai Institute of Materia Medica (SIMM) of Chinese Academy of Sciences, and ZHANG Jinyong from the National Engineering Research Center for Immunological Products, unveiled a rationally designed sulfonium-modified lipoglycopeptide, BD-V-2, which combats resistant Gram-positive pathogens through a dual mechanism involving enhanced inhibition of cell wall and membrane, as well as downregulation of type VII secretion system (T7SS) proteins.
Inspired by structures of approved lipoglycopeptides (oritavancin, telavancin) and recent potent analogs, the researchers synthesized three new vancomycin derivatives (BD-V-1, BD-V-2, BD-V-3) incorporating a sulfonium group and a hydrophobic biphenyl motif. Despite their hydrophobic features, all candidates demonstrated good aqueous solubility, likely due to the sulfonium group. Among them, BD-V-2 emerged as the most promising molecule based on in vitro potency.
Minimum inhibitory concentration (MIC) assays showed that BD-V-2 displayed dramatically improved activity, ranging from 2 to 7 orders of magnitude higher than vancomycin, against a panel of resistant Gram-positive clinical isolates, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate S. aureus (VISA), and vancomycin-resistant Enterococcus (VRE). BD-V-2 also demonstrated broad-spectrum activity, low cytotoxicity, and minimal hemolytic potential.
Pharmacokinetic (PK) studies showed that BD-V-2 outperformed vancomycin in several parameters. In lethal sepsis models caused by MRSA and VRE, BD-V-2 provided superior in vivo protection. Remarkably, a single 2.5 mg/kg dose of BD-V-2 achieved 100% survival in a VRE systemic infection model, highlighting its potent therapeutic potential even in severe infections with limited treatment options.
Mechanistically, BD-V-2 utilizes multiple pathways to fight bacteria. The sulfonium moiety, bearing a positive charge, enhances vancomycin’s interaction with bacterial membrane by binding to phosphatidylglycerol (PG), which increases membrane permeability and causes depolarization. Meanwhile, BD-V-2 significantly boosts inhibition of peptidoglycan synthesis, leading to accumulation of Park’s nucleotide intermediates. Structural studies suggested that BD-V-2 engages lipid II at two distinct sites that are not targeted by vancomycin, thus achieving dual membrane–cell wall inhibition, which is an approach that lowers resistance development risk.
Beyond direct antibacterial activity, the research team discovered two novel mechanisms. First, BD-V-2 self-assembles into micelles in aqueous solution, as shown by transmission electron microscopy (TEM) and dynamic light scattering (DLS) in collaboration with WU Zhenyong’s group from SIMM. Second, proteomic analysis revealed that BD-V-2 suppresses expression of all known proteins in the T7SS of S. aureus USA300, representing the first report of antivirulence activity in a glycopeptide derivative.
These discoveries position BD-V-2 as a next-generation antibiotic candidate with both potent antibacterial and antivirulence activity. Its multipronged mechanism offers fresh insights for future design of glycopeptide antibiotics to combat superbugs and prepare for AMR emergencies.
Link: https://pubs.acs.org/doi/10.1021/jacs.4c18630
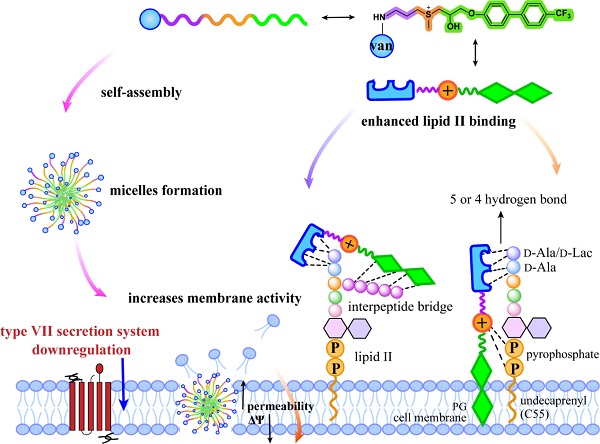
Schematic Illustration of the Multiple Mechanisms of BD-V-2 (Image by GUAN’s group)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




