Study Reveals Metabolic Impact and Clinical Relevance of Intact N-Glycosylation in Colorectal Cancer
Colorectal cancer (CRC) is a leading global malignancy, with tumor progression driven by complex metabolic reprogramming, including aberrant N-glycosylation patterns that play a pivotal role in tumor development.
Glycosylation is a post-translational modification in which carbohydrate chains form glycosidic bonds with reactive groups on specific amino acid side chains under catalytic activity of glycosyltransferases, resulting in covalent attachment of glycans to proteins. As one of the most prevalent and intricate protein modifications, glycosylation plays a vital role in biological regulation. Based on linkage between glycans and amino acids, glycosylation can be classified into O-linked and N-linked glycosylation.
Growing evidence indicates that protein glycosylation undergoes significant alterations during CRC initiation, progression, metastasis, and invasion. N-glycosylation is particularly critical for protein folding, stability, intracellular trafficking, cell-cell interactions, cellular differentiation, and immune responses. Dysregulated N-glycosylation has been closely associated with tumorigenesis, malignant transformation, metastasis, and invasion. Elucidating the molecular mechanisms by which N-glycosylation drives CRC development, along with identifying novel therapeutic targets, holds significant promise for advancing drug discovery and clinical treatment strategies for CRC. However, the metabolic and functional roles of N-glycosylation in CRC remain incompletely understood.
In a study published in Advanced Science on April 26, a research team led by HUANG He from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, in collaboration with LI Qunyi from Huashan Hospital of Fudan University, and MEI Qi from Tongji Hospital affiliated with Tongji Medical College of Huazhong University of Science and Technology, employed comprehensive intact N-glycoproteomic to elucidate the functional role of N-glycosylation in CRC metabolism and its clinical implications,
In this study, the researchers performed an in-depth proteomic and intact glycopeptide (IGP) analysis of 45 CRC tumor tissues and their matched normal adjacent tissues (NATs), identifying 7,125 IGPs corresponding to 704 glycoproteins. By analyzing IGP expression profiles and structural characteristics, the team constructed a glycan-glycosylation site-protein function association network, revealing N-glycosylation-driven functional dysregulation in CRC. Furthermore, the researchers developed an arithmetic model integrating differential expression of various N-glycans, which effectively distinguished tumors from NATs and reflected metabolic reprogramming in cancer. The study also identified Chloride Channel Accessory 1 (CLCA1) and Olfactomedin 4 (OLFM4) as potential metabolic biomarkers for CRC diagnosis, with immunohistochemistry and Cox regression analyses validating their prognostic utility. Notably, the research highlighted the critical role of site-specific N-glycosylation at N196 of adipocyte plasma membrane-associated protein (APMAP) in CRC progression, providing a potential therapeutic target.
These findings provided new insights and potential biomarkers for CRC diagnosis, prognosis, and targeted therapy, and offered valuable insights into the metabolic role of N-glycosylation in CRC, advancing biomarker discovery, enhancing metabolic-based diagnostic precision, and improving personalized therapeutic strategies targeting cancer metabolism.
DOI: 10.1002/advs.202415645
Link: https://doi.org/10.1002/advs.202415645
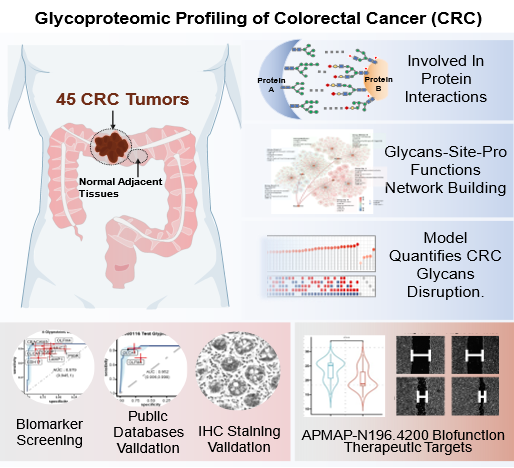
The regulatory role of glycosylation in CRC tumorigenesis and progression. (Image by Huang Lab)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




