Hedgehog-Interacting Protein Drives Lung Maturation and Confers Resistance to Chronic Respiratory Diseases
Alveologenesis is the developmental window when most lung gas-exchange surface forms. In humans, this process begins around prenatal week 36 and continues after birth, with new alveolar forming and maturing through adolescence. In mice, alveologenesis starts around postnatal day 3 (P3) and continues through P39. This process transitions from a “classical” phase (P3–P14), during which secondary septa subdivide saccules to dramatically expand alveolar surface area, to a “continued” phase (P15–P39) where coordinated lung growth and thoracic expansion that further increase exchange capacity.
Myofibroblasts in alveoli play a critical role in secondary septa formation during classical alveologenesis. These cells typically regress as lung development progresses beyond this phase. Dysregulation of myofibroblast formation and disappearance impairs alveologenesis. Disrupted alveologenesis leads to bronchopulmonary dysplasia (BPD), the most common and severe chronic lung disease in premature infants.
BPD is characterized by simplified alveolar architecture and abnormal accumulation of myofibroblasts, leading to reduced breathing capacity. Infants with BPD often require extensive and in-hospital support, increasing both family and social medical burden. Importantly, survivors of BPD face a substantially elevated risk of developing chronic obstructive pulmonary disease (COPD)/emphysema, which is marked by alveolar destruction and airspace enlargement. However, the molecular cascade linking early-life alveolar injury to adult emphysematous pathology remains largely elusive, and both BPD and COPD/emphysema lack targeted and effective therapies.
In a study published in Science Advances on May 7, a collaborative research team led by WANG Chaoqun from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, delineated a unifying mechanism by which Hedgehog-Interacting Protein (HHIP), a genetic susceptibility factor for both BPD and COPD/emphysema, regulats alveolar formation and long-term lung health. The study demonstrated that administration of recombinant HHIP protein in mouse models not only mitigates hallmark features of neonatal BPD but also prevents later development of COPD/emphysema changes in adulthood, positioning HHIP as both a key developmental regulator and a promising therapeutic candidate.
To uncover HHIP’s role in alveologenesis and disease, the researchers employed an integrative strategy combining lineage-tracing mouse models, high‐resolution single‐cell RNA sequencing (scRNA-seq), and different Hhip knockout mouse models. Lineage tracing revealed that alveolar myofibroblasts do not simply undergo apoptosis following alveolar maturation, but instead pass through a distinct “myofibroblast transition” marked by a phenotypic switch from α-smooth muscle actin (SMA)-positive to SMA‐negative states. Spatiotemporal analysis revealed a pronounced peak of HHIP expression in lung mesenchymal cells coinciding with alveolar septation and concurrent suppression of Hedgehog pathway activity.
In conditional Hhip knockout mice, aberrant activation of Hedgehog-IGF1 signaling arrested myofibroblast transition, resulting in persistent SMA-positive cells, induction of cell senescence programs in alveolar epithelial stem cells, and ultimately simplified alveolar structures, which recapitulated core BPD pathology. Strikingly, neonatal delivery of recombinant HHIP protein restored proper Hedgehog inhibition, rescued myofibroblast transition, and normalized alveolar development. Moreover, the treatment sustained protection into adulthood, blocking progressive airspace enlargement and preventing COPD/emphysema changes.
This work carried several far‐reaching implications. First, it established a molecular bridge connecting early‐life BPD with adult‐onset COPD/emphysema, underscoring how perturbations in developmental signaling pathways can predispose individuals to chronic disease decades later.
Second, by pinpointing HHIP as a master regulator of mesenchymal-epithelial crosstalk during alveologenesis, the research highlighted the critical role of timely modulation of Hedgehog-IGF1 activity in ensuring normal lung maturation and lifelong respiratory health. Finally, the findings opened a new therapeutic avenue for early developmental intervention to prevent later chronic lung disease. Notably, this study may inform strategies for other developmental disorders with late‐onset sequelae, offering a blueprint for harnessing developmental biology to combat chronic disease.
Link: http://doi.org/10.1126/sciadv.adu2958
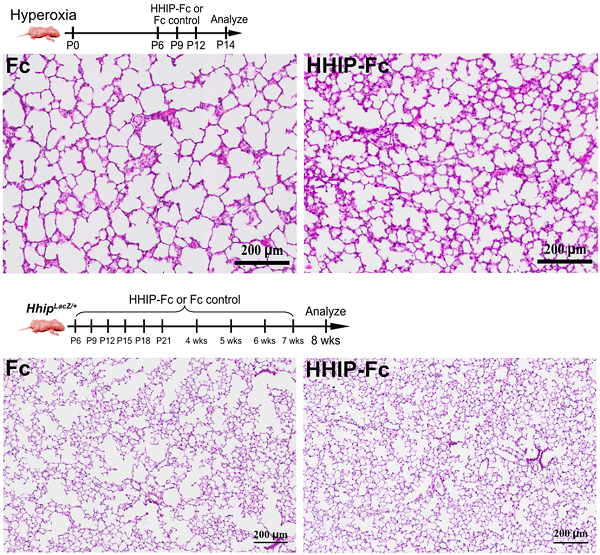
Administration of recombinant HHIP-Fc protein significantly ameliorated neonatal BPD (Upper) and adult COPD/emphysema (Lower) phenotypes, restoring alveolar density in animal models. (Image by YE Datian)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




