Researchers Circumvent “On-Target Apoptotic Toxicity” in RIPK3 Inhibitors via Conformational Regulation Strategy
RIPK3 is a key kinase that drives necroptosis—a programmed, pro-inflammatory form of cell death implicated in conditions such as sepsis, viral pneumonia, and neurodegenerative diseases. Owing to its central role in this pathway, RIPK3 has attracted significant interest as a therapeutic target. However, drug development efforts have been hampered by “on-target apoptotic toxicity”, where inhibitor binding unexpectedly triggers apoptosis. This paradoxical effect remains a major barrier to the clinical application of RIPK3 inhibitors.
In a study published in Nature Communications on May 8, a research team led by Prof. XU Yechun and Prof. ZHAO Qiang from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, reported a new class of selective RIPK3 inhibitors that effectively suppress necroptosis while avoiding apoptosis through engagement of a distinct, previously unrecognized binding site.
This study was prompted by previous findings that R69H mutation in RIPK3 can suppress apoptosis induced by the well-known inhibitor GSK'872. To elucidate the underlying mechanism, the researchers solved crystal structures of both wild-type and R69H-mutant RIPK3 bound to GSK'872. These structures revealed that the mutation disrupts key hydrogen bonds at the dimer interface, thereby weakening kinase domain dimerization.
Building on this insight, the researchers employed a structure-based design strategy. They noted that R69H mutation lies near the αC-helix and that the thiazole ring of GSK'872 points toward this region. This observation led to the hypothesis that introducing hydrophobic substituents to the thiazole ring may mimic conformational disruption caused by the mutation and thereby inhibit dimer formation. Based on this hypothesis, the researchers designed and synthesized four novel compounds, LK01001, LK01002, LK01003, and LK01045. These compounds exhibit potent inhibition of RIPK3 and robust anti-necroptotic efficacy in cellular models. Unlike GSK'872, these compounds do not induce caspase-3 activation even at concentrations up to 50 μM, representing a substantial improvement in safety profile.
Crystal structure of RIPK3 in complex with LK01003 revealed that its cyclopentyl group induces a conformational rearrangement in the αC-helix and DFG motif, allowing the compound to engage a previously unexplored hydrophobic pocket. The binding mode of LK01003 destabilizes the R69 side chain and disrupts key hydrogen bond interactions at the dimer interface, thereby mimicking conformational consequences of R69H mutation and blocking dimerization. Notably, LK01003 exhibits outstanding selectivity, with an S (35) score of 0.005 at 1 μM across a panel of 379 kinases—outperforming the selectivity of GSK'872.
The researchers also applied this conformation-regulation strategy to other RIPK3 inhibitors known to induce apoptotic toxicity, including PP2 and Zharp-99. By modifying these compounds to interact with newly identified allosteric site, they successfully created analogs that retained kinase inhibition and necroptosis-blocking activity while eliminating pro-apoptotic effects.
This work not only provided a new class of RIPK3 inhibitors with improved safety and selectivity, but also opened new avenues for kinase drug discovery by demonstrating how targeting non-catalytic, conformationally dynamic regions can overcome long-standing challenges in drug design.
DOI: 10.1038/s41467-025-59432-8
Link: https://doi.org/10.1038/s41467-025-59432-8
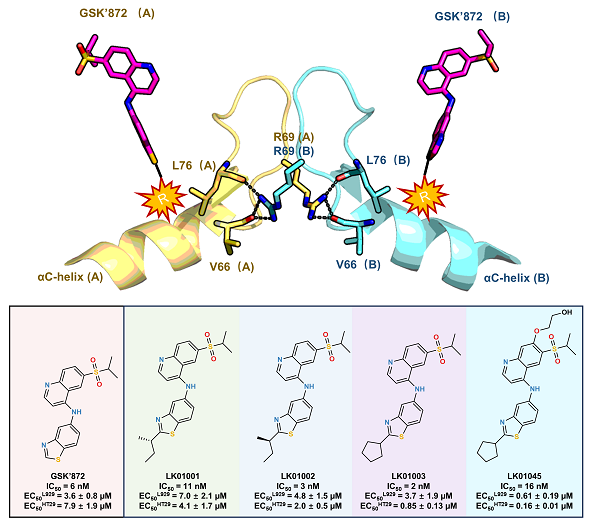
Structure-based design strategy for non-apoptotic RIPK3 inhibitors and their biological activity (Image by SU Haixia)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




