Researchers Reveal Activation Mechanism of Prostaglandin E2 Receptor EP1
Prostaglandin E2 (PGE2), a bioactive lipid derived from arachidonic acid, mediates a broad range of physiological processes through four G protein-coupled receptor (GPCR) subtypes: EP1–EP4. While high-resolution structures of EP2, EP3, and EP4 have been resolved, EP1 remained structurally uncharacterized due to its intrinsic instability, hindering detailed understanding of its Gq-mediated signaling.
In a study published in PNAS on May 15, a research team led by Eric H. Xu (XU Huaqiang) and XU Youwei from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, reported cryo-electron microscopy (cryo-EM) structure of the human EP1 receptor in complex with PGE2 and the heterotrimeric Gq protein. This work completed structural atlas of EP receptor family and revealed EP1-specific mechanisms of ligand recognition and signal transduction.
To overcome the instability of EP1, the team employed a multi-pronged engineering strategy, including BRIL fusion, truncation of flexible loops, incorporation of a mini-Gq chimera, and NanoBiT-assisted complex stabilization. Using single-particle cryo-EM, they resolved structure of the EP1–PGE2–Gq complex at 2.55 Å resolution, enabling detailed analysis of both ligand binding and G protein coupling interfaces.
A key observation was that activation of EP1 induces a modest outward shift of transmembrane helix 6 (TM6) by approximately 12°, which is notably smaller than the nearly 18° displacement seen in EP2–EP4, suggesting a subtype-specific activation mechanism. The researchers also identified a unique constellation of residues- S421.42, H882.54, G922.58, F3347.36, that form a distinct binding motif for PGE2, absent in other EP receptors.
Functional assays confirmed that these residues are critical for ligand-induced activation. Notably, EP1 diverges from canonical class A GPCR motifs: it lacks conserved DRY sequence and features an unusual cysteine at position 3.51, further highlighting its unique signaling profile.
On intracellular side, the EP1 receptor engages Gq protein through both conserved and receptor-specific interactions. Residues such as R63ICL1, E2946.32, and Q2986.36 contribute to the precise orientation of Gα5 helix within the cytoplasmic cavity. Additional compensatory interactions, such as those involving S692.35-appear to stabilize G protein coupling, compensating for missing contacts found in related receptors FP and TP.
These findings not only completed the structural framework of PGE2 signaling through its receptor family, but also provided a blueprint for developing selective EP1-targeted therapies. Given EP1’s involvement in pain, cardiovascular disease, and certain cancers, structure-based drug design informed by this study holds significant translational potential.
DOI: doi/10.1073/pnas.2423840122
Link: https://doi.org/10.1073/pnas.2423840122
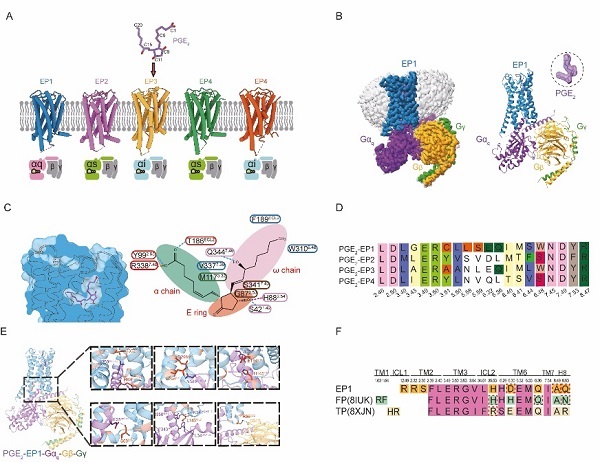
Cryo-EM structure of the human EP1 receptor bound to PGE2. (Image by XUE Meng)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




