Researchers Disrupt cGAS-DNA Phase Separation by De Novo Design of Allosteric Inhibitors: A Potential Therapeutic Strategy
Cyclic GMP-AMP synthase (cGAS), a key mediator of the cGAS-STING DNA sensing pathway that triggers type-I interferon responses, plays a crucial role in innate immunity and has been implicated in the pathogenesis of autoimmune diseases, inflammatory conditions, neurodegenerative diseases, and COVID-19. Upon sensing cytosolic DNA, cGAS undergoes liquid-liquid phase separation (LLPS) via multivalent interactions with DNA, forming condensates that concentrate cGAS and enhance its activation. However, traditional orthosteric inhibitors often fail to effectively inhibit concentrated cGAS within these condensates caused by phase separation, leading to a significant drop in cellular potency compared to enzymatic inhibitory activity.
In a study published in Nature Communications, a research team led by XU Yechun from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences (CAS) reported the discovery of an allosteric site adjacent to cGAS’s activation loop—a region critical for DNA binding and conformational activation. By targeting this site, they designed first-in-class protein condensation inhibitors (PCIs), represented by compounds XL-3156 and XL-3158. These inhibitors simultaneously occupy both the allosteric and orthosteric sites, locking the activation loop in a closed conformation and thereby attenuating cGAS-DNA interactions as well as condensate formation. Co-crystal structure determination revealed that XL-3156 binds to cGAS in a "butterfly wings" configuration.
In more detail, the breakthrough emerged from crystallographic analysis of RU.521 bound to human cGAS, revealing an unexpected allosteric binding site near the activation loop. Structure-based drug design yielded XL-3156, which mimicked the "butterfly wings" binding mode of RU.521 enantiomers. Biochemical and cellular assays demonstrated that XL-3156 and its derivative XL-3158 disrupted cGAS-DNA interactions, prevented LLPS condensate formation, and triggered a morphological shift from solid-like aggregates (LSPS) to liquid droplets (LLPS) at the molecular level.
DNA condensation assays with activation loop mutants and molecular dynamics simulations confirmed that PCI’s potency depended on its interactions with the conserved residues of activation loop. Unlike orthosteric inhibitors, PCIs overcome the cellular potency drop by preventing condensate formation.
While XL-3158 showed weaker enzymatic inhibition than G150, it achieved comparable cellular efficacy by maintaining low concentrations of free cGAS. PCIs also exhibited cross-species activity, inhibited both human and mouse cGAS, and synergized with G150 as they blocked condensation while G150 targeted free cGAS. In a cerulein-induced acute pancreatitis mouse model, oral XL-3158 reduced pancreatic damage, serum amylase/lipase levels, and pro-inflammatory cytokines.
These findings underscore the therapeutic potential of targeting protein condensation and highlight the importance of evaluating inhibitors in cellular contexts. Importantly, the strategy of developing PCIs to enhance the cellular efficacy of inhibitors may apply to other proteins that undergo phase separation, opening up broader avenues for drug discovery and development. This study not only reveals a previously unrecognized mechanism for cGAS inhibition but also highlights the therapeutic potential of targeting phase separation to regulate protein function.
DOI: 10.1038/s41467-025-60297-0
Link: https://doi.org/10.1038/s41467-025-60297-0
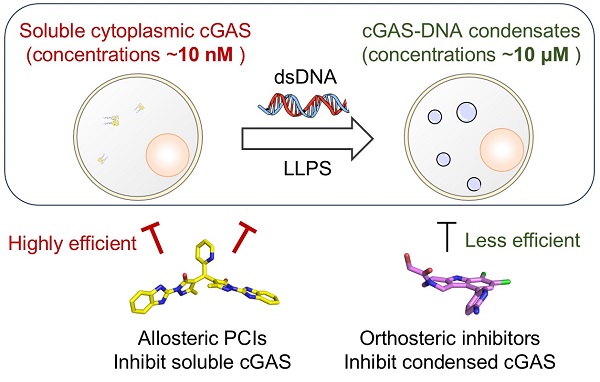
Schematic illustration of cellular effects mediated by different classes of inhibitors (Image by ZHAO Wenfeng)
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




