Researchers Reveal How Macrophages Deploy “Fishing Nets” to Catch Distant Bacteria
In a study published in The EMBO Journal, a research team led by JIU Yaming from the Shanghai Institute of Materia Medica (SIMM) of Chinese Academy of Sciences (CAS), along with collaborators, demonstrated that DLPs form in exposure to severe Gram-negative infection within a few hours. Using cultured cells and live mice model, they showed that macrophages equipped with DLPs reduced bacterial burdens. The study also mapped the molecular pathway that drives DLP construction, revealing a sequence of signals that begins with bacterial lipopolysaccharide and ends with a burst of actin-driven growth and vimentin-driven stabilization.
To capture the process in real time, the researchers combined three complementary approaches. First, they infected human THP-1 macrophages with fluorescence-labelled Salmonella enterica serovar Typhimurium and followed the cells on a spinning-disk confocal microscope. Within hours, round macrophages began to sprout slender tubes that branched like miniature dendrites. By 6 hours, roughly 30 % of the population had transformed into macrophages with mature DLPs. Second, the team built a microfluidic chip that funnels bacteria toward macrophages through 10-micrometre channels. DLPs consistently grew toward the channels that contained Salmonella. When bacteria were physically separated from macrophages by a 0.4-micrometre membrane, no DLPs formed, proving that direct contact is required. Third, the group used two-photon intravital microscopy to peer into the peritoneal cavity of living mice. After intraperitoneal infection, peritoneal macrophages anchored to the mesothelium extended DLPs deep into the fluid, capturing motile Salmonella in situ.
The molecular dissection began with a simple observation: only Gram-negative bacteria triggered DLPs. Gram-positive Listeria or Bacillus failed, as did purified peptidoglycan or lipoteichoic acid. Purified lipopolysaccharide (LPS), however, reproduced the effect. LPS binds to Toll-like receptor 4 (TLR4) on macrophage membranes. When the team deleted TLR4 with CRISPR-Cas9 or blocked it with the drug TAK-242, DLP formation dropped to baseline. Downstream, LPS-TLR4 signalling activated NF-κB, which entered the nucleus and turned on the gene encoding ARHGEF3, a guanine-nucleotide-exchange factor specific for the small GTPase RhoA. ARHGEF3 then relocated to the base of each nascent DLP and loaded RhoA with GTP, initiating cycles of actin polymerisation. Live imaging with SiR-actin revealed a three-step cycle: a rapid growth phase driven by actin assembly, a steady plateau, and a collapse phase in which bundles disassembled. The intermediate filament protein vimentin accumulated inside each DLP and acted as a buttress; without vimentin, the protrusions repeatedly initiated but immediately retracted, forming only unstable blebs.
Functionally, compared with round macrophages lacking DLPs, cells with DLPs internalized twice as many bacteria at multiplicities of infection. Moreover, genetic deletion or pharmacological inhibition of either ARHGEF3 or vimentin blocked DLP formation and subsequently blunted the macrophages’ ability to fight infection, confirming that DLPs - not some other change - were responsible for the improved clearance. To test the biological payoff, in a mouse model of Salmonella infection, the team reinfused animals with macrophages that had been pre-stimulated in vitro to generate abundant DLPs. Bacterial burdens in the peritoneal cavity and liver dropped markedly, and clinical signs of infection eased, confirming that DLPs are critical for host defense.
This study not only reveals, for the first time, a morphological defense mechanism mounted by macrophages during severe bacterial infection, but also offers a potential therapeutic target against drug-resistant Gram-negative pathogens. The discovery of DLP rewrites the textbook picture of macrophage plasticity. Classical filopodia and lamellipodia are short-lived, actin-only structures that probe the immediate perimeter. DLPs are longer-lived, composite structures that enlist both actin and vimentin to search distant space. Because the TLR4-ARHGEF3-RhoA pathway is druggable, the work suggests new ways to boost innate immunity against the rising tide of multidrug-resistant Gram-negative bacteria. Future studies will ask whether other immune cells, such as microglia or dendritic cells, can also weave these nets, and whether synthetic biology can engineer DLPs with customized ligands to snare specific pathogens.
DOI: 10.1038/s44318-025-00515-z
Link: https://www.embopress.org/doi/full/10.1038/s44318-025-00515-z
Keywords: macrophage morphological defence, dendrite-like pseudopods, bacterial infection
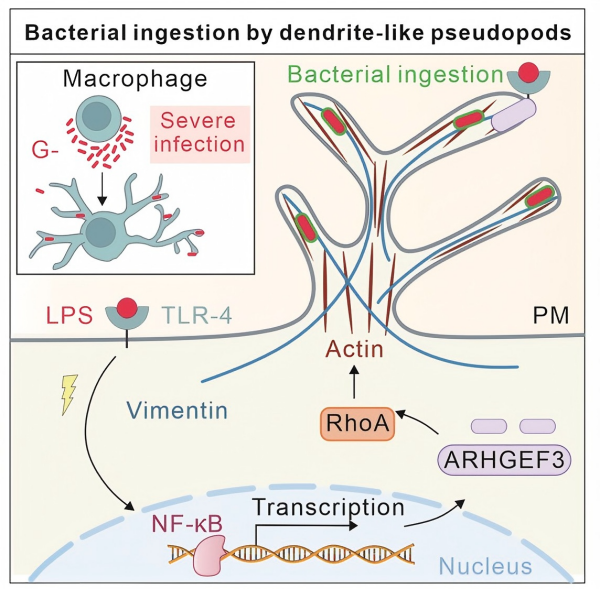
The mechanism and function of macrophages producing DLPs (Image by FAN Changyuan)
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




