Researchers Discover Potent Oral Sialylation Inhibitor That Enhances Immunotherapy for Pancreatic Ductal Adenocarcinoma
Aberrant sialylation is a hallmark of cancer, and plays an important role in cancer progression. Sialic acids on tumor cell surfaces link to Siglec receptors on immune cells, suppressing immune responses and supporting immune evasion. Consequently, intervening in the sialylation process is considered a promising direction for developing novel cancer treatment strategies, with both biomacromolecule and small-molecule drugs targeting this pathway showing significant development potential.
In a study published in ACS Central Science, a research team led by YU Biao from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and GONG Likun from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, discovered a most potent sialylation small-molecule inhibitor to date, demonstrating significant immune microenvironment activation and antitumor efficacy.
This study utilized pancreatic ductal adenocarcinoma (PDAC) cells, a model exhibiting a typical highly sialylation phenotype, to establish a high-throughput screening platform. Through this platform, the small molecule compound Y-320 was identified, which could significantly reduce sialylation levels on the surface of tumor cells. Further experiments demonstrated that Y-320 broadly inhibited α-2,3/2,6 sialylation modifications on the PDAC cell surface, with an IC₅₀ of approximately 200 nM, over 300 times more potent than the classic pan- sialylation inhibitor P-3Fax-Neu5Ac. Molecular docking analysis suggested Y-320 mostly exerted its inhibitory effect by competitively occupying the substrate-binding pockets of multiple sialyltransferases.
In multiple in vivo models, Y-320 inhibited tumor growth and altered the tumor immune microenvironment. Y-320's antitumor activity was linked to a synergy between CD8⁺ T cells and macrophages, as indicated by immune cell analysis. Further animal studies showed that the combination of Y-320 and anti-PD-1 antibodies has a potent synergistic therapeutic effect. The tumor suppression effect of the combination was significantly higher than that of monotherapy, indicating that Y-320 could effectively reverse PDAC resistance to immune checkpoint blockade therapy.
This study not only provides a small-molecule tool for investigating the biofunction of sialylation but also validates the potential of sialylation inhibition as a novel glyco-immune checkpoint strategy for treating PDAC and other immunotherapy-resistant tumors. It further demonstrates that Y-320 holds promising prospects for clinical translation.
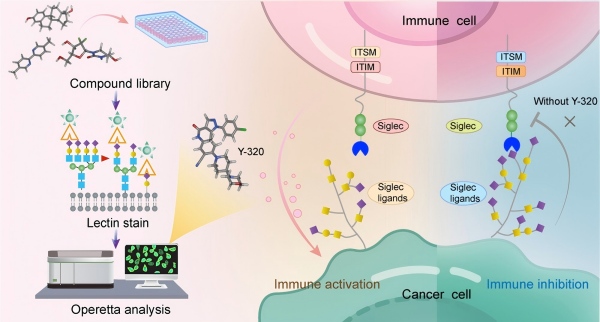
Mechanism of action diagram. (Image by Gong Likun’s laboratory)
Link: https://pubs.acs.org/doi/10.1021/acscentsci.5c00939
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




