Researchers Unveiled Conformation-Selective “Valve Model” for Norepinephrine Transporter Regulation
In a study published in Cell, researchers from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences and the Lingang Laboratory, together with collaborators, have unveiled a conformation-selective allosteric regulation mechanism of the norepinephrine transporter (NET), which establishes a new “valve model” that explains how NET recognizes and responds to structurally diverse inhibitors, offering a conceptual framework for the design of next-generation antidepressants.
NET is a crucial member of the monoamine transporter family responsible for the reuptake of norepinephrine and dopamine to maintain neurotransmitter homeostasis. Dysfunction of NET is linked to depression and attention-deficit disorders, yet how it is precisely modulated by various drugs has remained poorly understood. Building on their previous Nature (2024) study that revealed NET dimerization and antidepressant binding, the team determined three high-resolution cryo-EM structures (2.44–2.52 Å) of human NET bound to different antidepressants—all captured in the inward-open conformation.
Structural comparisons uncovered a new inward-open-specific allosteric site (S3) and two key phenylalanine residues (F72 and F329) that act like a dynamic valve, partitioning the inward cavity into two asymmetric chambers. Depending on the inhibitor’s shape and size, the valve guides ligand binding to distinct subregions, explaining the transporter’s conformational selectivity and diversity in drug recognition.
Leveraging this model, the researchers performed virtual screening of over 520,000 compounds, leading to the discovery of F3288-0031, a potent and selective NET inhibitor with antidepressant-like activity in mice and favorable pharmacokinetic and safety profiles. This multidisciplinary “structure–computation–function–validation” strategy establishes a new paradigm for structure-based drug discovery targeting monoamine transporters.
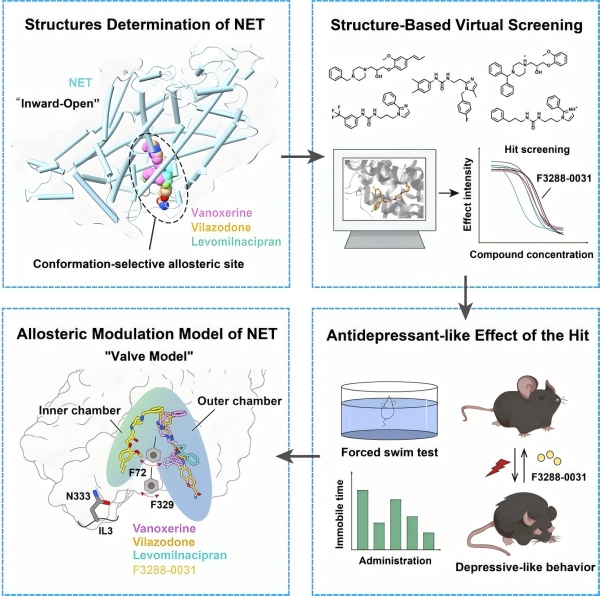
Structure-based discovery of a conformation-selective allosteric inhibitor of NET (Image by SIMM)
DOI: 10.1016/j.cell.2025.10.002
Link: https://doi.org/10.1016/j.cell.2025.10.002
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




