Researchers Identify the “Northwest Passage” Mechanism of Bile Acid Transport
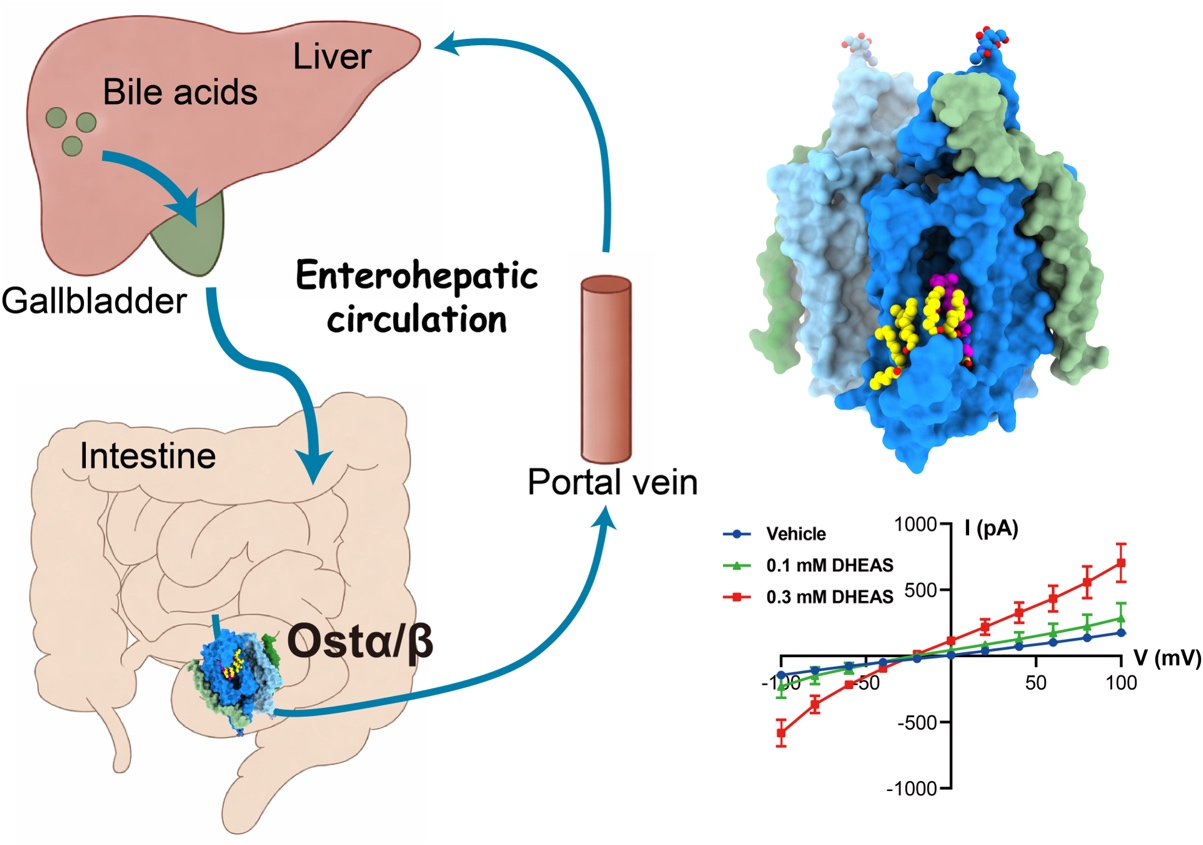
Bile acids are essential for digestion, metabolism, and hormonal signaling. Their circulation between the liver and intestine, known as enterohepatic circulation, depends on a coordinated network of membrane transporters. However, the critical molecular mechanism by which bile acids are exported from enterocytes into the bloodstream has remained unclear—prompting one reviewer to describe it as the “Northwest Passage” of bile acid transport: a pathway long presumed to exist but elusive to map.
Now, a research team led by Eric H. Xu (XU Huaqiang) from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, together with MA Xiong from Renji Hospital, has clarified this molecular mechanism. Using cryo-EM structure determination, molecular dynamics simulations, and electrophysiological analyses, the team determined how Ostα/β transports bile acids and why it differs fundamentally from previously characterized carriers. The study was published in Nature on January 28.
Bile acid transport in hepatocytes is accomplished by sodium-coupled or facilitative transporters that mediate bile acid uptake at the sinusoidal membrane, while ATP-binding cassette (ABC) transporters drive bile acid export at the canalicular membrane. Such transport logic was assumed to exist in enterocytes and other bile acid-transporting epithelia. But in 2004, the heterodimeric organic solute transporter Ostα/β was identified as the major basolateral bile acid exporter. Nevertheless, its molecular mechanism remained a mystery.
In this study, the researchers expressed and purified the human Ostα/β complex in mammalian cells and solved its structure at resolutions ranging from 2.6–3.1 Å through single-particle cryo-electron microscopy. They discovered that Ostα/β assembled as a symmetric tetramer composed of two heterodimers. Furthermore, each Ostα subunit formed a unique seven-transmembrane fold, which is “augmented” by a single transmembrane helix of Ostβ. This architecture explains why Ostα/β is not classified within known transporter families.
Structural analysis revealed a lateral substrate-binding groove embedded within the membrane, which was stabilized by a cysteine-rich loop that underwent extensive palmitoylation. The lipid modifications created a hydrophobic environment that accommodated amphipathic substrates. Structures bound to taurolithocholic acid and dehydroepiandrosterone sulfate showed how charged residues within the groove interacted with negatively charged substrate groups, conferring specificity.
Moreover, the researchers identified a hydrophilic tunnel extending from the binding groove toward the extracellular side of the transporter. Molecular dynamics simulations and electrophysiological recordings showed that substrates moved through this pathway in a voltage-dependent manner, directly converting bile acid flux into an electrical signal by using the intrinsic charge of cholic acid. These results provided a direct, quantitative readout of bile acid transport by recording transporter-associated currents, thus linking structure to transport in real time and with polarity control.
Together, the data support a model in which Ostα/β functions as a facilitative carrier whose transport direction is set by the combined electrochemical gradient of its substrates. Ostα/β mediates bidirectional flux with directionality shaped by substrate concentration gradients, membrane potential, and electrostatic interactions within the binding pocket. As a result, membrane voltage is not a passive background variable but an active determinant that biases transport toward export- or import-favored modes under physiological conditions.
This study goes beyond bile acid biology to show that Ostα/β and the TMEM184 family of proteins are likely orphan transporters rather than receptors based on their structural similarity and thus may share transport mechanisms. These results open up new avenues for studying poorly characterized membrane proteins and understanding how lipid environments tune transporter function.
DOI: 10.1038/s41586-025-10029-7
Article link: https://www.nature.com/articles/s41586-025-10029-7
Keywords:Bile acid transport; Cryo–electron microscopy; Voltage dependent
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




