Chemokine Receptor CCR5 Structures Uncover Mechanism of Chemokine Recognition and Receptor Activation
Chemokine receptors regulate the migration of immune cells and serve as valuable drug targets for the treatment of cancer, AIDS and inflammatory diseases. The chemokine receptor CCR5 is involved in inflammation, tumor and pathogen infection. However, key factors of CCR5 that govern chemokine recognition and receptor activation have not been elucidated yet.
In a study published in Nature Communications, a research team led by WU Beili, ZHAO Qiang and XU Yechun from Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences reported three cryo-electron microscopy structures of Gi1 protein-coupled CCR5 in a ligand-free state and in complex with the chemokine MIP-1α or RANTES, as well as the crystal structure of MIP-1α-bound CCR5.Combining the high-resolution crystal structure of the CCR5-MIP-1α complex with disulfide bond cross-linking and molecular dynamics simulations, the researchers for the first time found molecular details of the binding mode between the CCR5 N terminus and a chemokine, giving key information on the specific recognition between the chemokine and its receptor.
The researchers compared structures of CCR5 in complex with different chemokines. They found that when bound to RANTES, CCR5 exhibited a more open ligand-binding pocket with its first and second transmembrane helices shifting outwards to accommodate the larger N terminus of RANTES, which suggested that plasticity of the transmembrane helical conformation provides a structural basis for CCR5 binding to different chemokines.
Besides, the researchers found that the N termini of MIP-1α and RANTES insert into the ligand-binding pocket within the receptor transmembrane helical bundle. They showed that by interacting with the receptor residue Y251, a key residue W248 is stabilized in an active conformation, which facilitates receptor activation, and that in the ligand-free state, the side chain of W86 in the receptor rotates by about 90 degrees to stabilize the receptor in the active conformation by forming interactions with Y108, Y251 and W248, thereby activating the receptor.
This study suggests the recognition of CCR5 from other endogenous chemokines, and unveils the molecular mechanism of chemokine signal transduction, laying foundation for targeted drug therapy against tumor, HIV and inflammation.
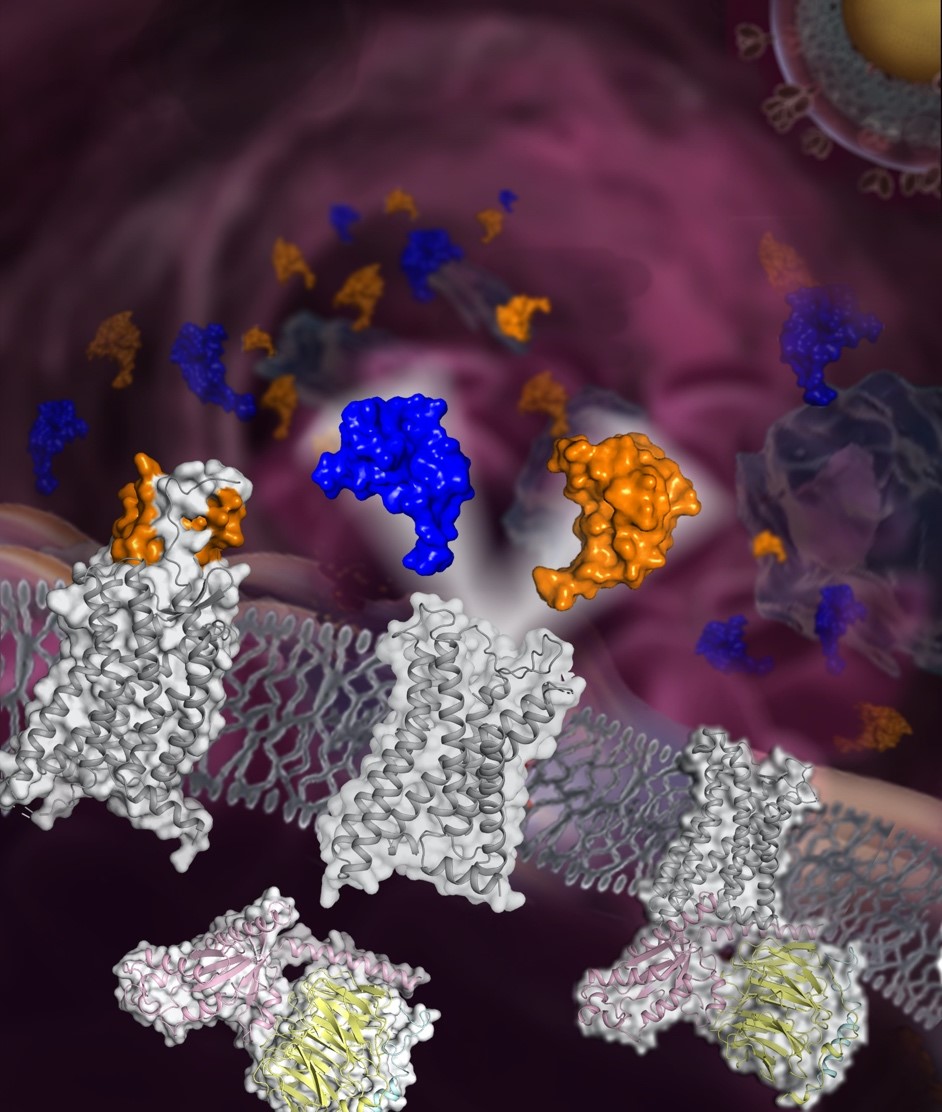
The schematic diagram of the chemokine receptor CCR5 structures (Image by WU’s laboratory at SIMM)
Links
DOI:10.1038/s41467-021-24438-5
Contact
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




