Researchers Reveal How Lipids and Water Molecules Regulate 5-HT Receptors
Serotonin, or 5-hydroxytryptamine (5-HT), is a kind of neurotransmitter. 5-HT can regulate multifaceted physiological functions such as mood, cognition, learning, memory, and emotions through 5-HT receptors. 5-HT receptors are a type of G protein-coupled receptor and can be divided into 12 subtypes in humans. As drug targets, they play a vital role in the treatment of schizophrenia, depression, and migraine.
However, the structural and functional mechanisms of 5-HT receptors have been largely unknown.
In a study published in Nature on March 24, Prof. H. Eric XU and Prof. JIANG Yi from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, together with Prof. ZHANG Yan from Zhejiang University, and their collaborators, have clarified the critical role of PtdIns4P and cholesterol in G-protein coupling and ligand recognition as well as the molecular basis of basal activity and the drug recognition mode of 5-HT receptors, by resolving the cryo-electron microscopy (cryo-EM) structures of five 5-HT receptor–Gi complexes.
These five 5-HT receptor–Gi complexes include three with 5-HT1A structures (one in the apo state, one bound to 5-HT, and one bound to aripiprazole, an antipsychotic drug), one with 5-HT1D bound to 5-HT, and one with 5-HT1E bound to the 5-HT1E- and 5-HT1F-selective agonist BRL-54443.
PtdIns4P is one of the major classes of phosphoinositides. In this study, the researchers first identified PtdIns4P as a major phospholipid at the 5-HT1A–G protein interface, which stabilizes the 5-HT1A-G protein complex.
They found that PtdIns4P is sandwiched between two cholesterol molecules surrounding the 5-HT1A receptor, therefore providing a structural basis for the modulation of 5-HT1A signaling by cholesterol and phospholipids.
Researchers also found several structured water molecules that form hydrogen bonds with the apo receptor within the orthosteric binding pocket. Water molecules mimic the polar functionalities of 5-HT in the active apo-5-HT1A–Gi complex, thus revealing the key role of water molecules in sustaining the basal activity of 5-HT receptors.
In addition, the researchers revealed the basis of ligand selectivity and drug recognition in 5-HT receptors. They identified residue at position 6×55 as a key determinant for the BRL-54443 and 5-CT selectivity of 5-HT receptors.
An outward shift of the extracellular end of TM7 in 5-HT1A stabilizes the quinolinone group of aripiprazole, resulting in 5-HT1A’s high selectivity for aripiprazole.
A cholesterol molecule was further found to be involved in the stabilization of the aripiprazole pocket and causes aripiprazole to have a higher binding affinity for 5-HT1A.
The observations in this study have wide implications for a mechanistic understanding of 5-HT signaling and for drug discovery targeting the 5-HT receptor family.
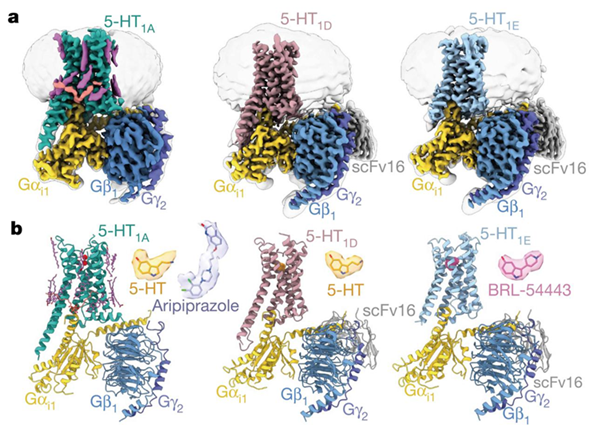
Cryo-EM structures of the 5-HT1A-Gi, 5-HT1D-Gi, and 5-HT1E-Gi complexes (image by H. Eric Xu’s group)
DOI:10.1038/s41586-021-03376-8
Links:https://dx.doi.org/10.1038/s41586-021-03376-8
CONTACT:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




