Stabilizing Vitamin D3 by Conformationally Selective Co-crystallization
Vitamin D3 is an example of chemically and physically unstable drug for osteomalacia and osteoporosis. The compound has an unstable conjugated triene group that undergoes topochemical reactions. Due to it unstability aspects, Vitamin D3 drug substance has to be stored under controlled conditions for storage and transportation according to USP 34, and the drug was rarely formulated as solid dosage form due to its undesired properties under solid-state.
Owning to its biological importance and it’s widely application in food, drug and nutrition industry, the stability of Vitamin D3 has been studied extensively in the literature, including chemical, photochemical and thermal aspects. However, to the best of our knowledge, there has been no effective method was developed to solve the stability challenge of this important drug yet.
Researchers from Prof.MEI Xuefeng’s lab in Shanghai Institute of Materia Medica, CAS reported the discovery of a four-membered square-shaped structure constructed with a set of collective hydrogen-bond interactions to result in the formation of co-crystals of 1a and 1b.
These co-crystals were formed through an unexpected conformationally selective crystallization process and were found to present superior physicochemical properties compared with VD3 itself.
Researchers have prepared two co-crystals of VD3 based on a four-membered square-shaped structure utilizing a set of weak, collective hydrogen-bond interactions. The co-crystallization process was found to be conformationally selective to give exclusively a topochemical stable form in the solid structures. The two co-crystals are isostrutural related and present superior chemical and physical stability comparing with VD3 alone.
The findings of this report may encourage applying its co-crystals over the current pure form in the future development of this important anti-osteoporosis drug, and may further facilitate the widely applications of VD3 in food and nutrition industry.
This work has been published on the Chem.Commun.
Full text:http://pubs.rsc.org/en/content/articlepdf/2014/cc/c3cc47747a
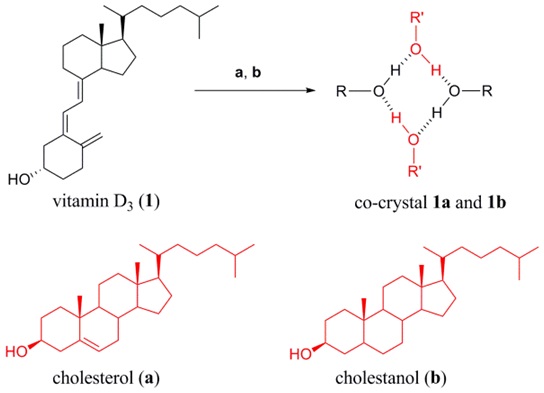
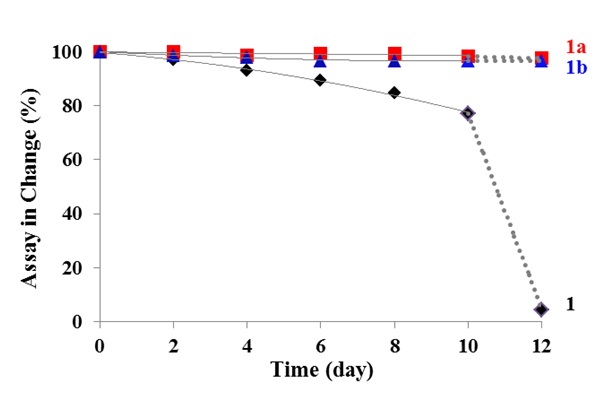
Figure 1.Chemical structures of vitamin D3 (1) and co-formers (Left) and changes of vitamin D3 assay values during storage at 40 °C/75% RH (Right)




