Pynegabine, a First Class Anti-epileptic Drug Targeting KCNQ Potassium Channel Approved for Clinical Study
Recently, a first class new anti-epileptic drug, pynegabine tablet, developed by NAN Fajun’s and GAO Zhaobing’s research groups at the Shanghai Institute of Materia Medica(SIMM), Chinese Academy of Sciences (CAS), has received the approval of State Food and Drug Administration(SFDA) for clinical trial.
Epilepsy is one of the most common and serious neurological disorders, with an overall prevalence of nearly 10 per 1000 worldwide, affecting approximately 10 million people in China.
The mainstay treatment of epilepsy is anti-epileptic drug. However, about one third of patients are resistant to available pharmacologic treatments, known as Drug-resistant epilepsy. Therefore, the urgent need to develop new antiepileptic drugs aiming at novel targets remains a clinical necessity.
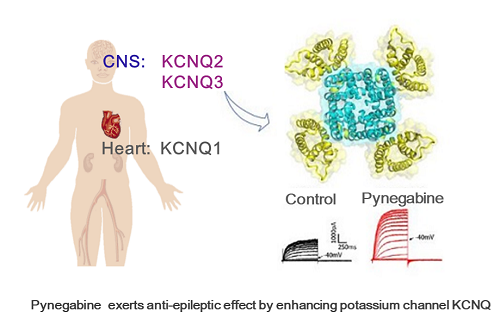
The global market for epilepsy drugs is nearly 20 billion dollars per year. Potassium channel KCNQ is a promising anti-epileptic target, and retigabine is the first and only anti-epileptic drug previously approved by FDA in 2011 with a mechanism of action that potentiates KCNQ-mediated potassium current.
Retigabine has significant clinical effects, especially for some refractory epilepsy and epileptic encephalopathy. However, some patients with long-term medication have serious dose-related side-effects such as skin and retinal pigmentation, mainly due to poor chemical stability and poor metabolic characteristics.
Retigabine received a black box warning in 2013, and it was discontinued from all markets in 2017 for commercial reasons. The international development of anti-epileptic drugs targeting KCNQ is in the ascendant, and the second-generation anti-epileptic new drug XEN1101, based on the structural optimization of retigabine, has been recently completed for Phase I clinical trials. Retigabine has also been picked up and headed for a quick makeover for refractory epilepsy in children.
Pynegabine, a new generation anti-epileptic drug candidate, targeting KCNQ potassium channel, overcomes retigabine’s unstable chemical structure and poor metabolic characteristics, and has fully independent intellectual property rights.
Considering the pharmacodynamic safety and metabolic characteristics following several rounds of structural optimization, NAN’s and GAO’s research groups have spent eight years on developing pynegabine as the anti-epileptic drug candidate.
Preclinical studies have shown that pynegabine improves upon chemical stability and brain distribution over retigabine. In addition, pynegabine not only reduces the risk of pigmentation but also shows greater potency than retigabine in multiple animal models including refractory epilepsy model.
Pynegabine, hopefully, will become a new anti-epileptic drug of Best/Only in-class KCNQ modulator with proprietary intellectual property rights and following further clinical trials. It may provide new drug treatment options for refractory epilepsy patients in the future. All these results show that pynegabine will have good development prospects.
The study was supported by ZHONG Dafang’s and CHEN Xiaoyan’s research groups (Center for Drug Metabolism and Pharmacokinetics Research of SIMM), CHEN Dongying research group (Center for Pharmaceutical Analysis and Solid-state Chemistry Research of SIMM), LI Yaping’s research group (Center for pharmaceutics Research of SIMM), Zhejiang University Center for Drug Safety Evaluation and Research, and National Shanghai Center for New Drug Safety Evaluation and Research. The project was also supported by Funds for Major Project on “Key New Drug Creation and Manufacturing Program” of the National Science and Technology of China, the Strategic Pilot Project in Science and Technology of CAS, Shanghai Municipal Science and Technology Commission, and the self-deployment project of the Institutes of Drug Discovery and Development of CAS.
Contact:
GAO Zhaobing (zbgao@simm.ac.cn)
(Credit: XU Haiyan; Editor:PAN Peihua)




