Scientists Discover Therapeutical Potential of Adoptive Treg Cell Transfer in Rescuing MIA-induced Behavioral Abnormalities
In a study published in Nature Neuroscience, Prof. ZHOU Zikai from Shanghai Mental Health Center , now a professor at Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, and the collaborators, reported a novel animal model of parasite-mimicking maternal immune activation (MIA), revealed immuno-neurological mechanisms underlying MIA-induced behavioral abnormalities and demonstrated therapeutic efficacy of adoptive transfer of regulatory T cells (Tregs) in rescuing phenotypes associated with immune alterations.
Epidemiologic evidence in humans has implicated a possible role for prenatal maternal infections and resultant immune activation, in the pathogenesis of neuropsychiatric disorders such as schizophrenia (SZ) and autism spectrum disorder (ASD) in offspring. Animal studies of MIA routinely used the bacterial mimetic lipopolysaccharides (LPS) and the viral mimetic polyinosinic:polycytidylic acid (poly(I:C)). These immune-activating agents directly target the innate immune system to induce MIA without reproducing full spectrum of immune responses normally induced by infectious pathogens, particularly the pathogen-specific adaptive cellular immune reactions mediated by T cells.
In this study, the scientists used the soluble tachyzoite antigen (STAg) from T. gondii to induce MIA in mice. They found that this approach induced profound and chronic dysregulation of immune profiling in the brain and peripheral circulation in adult offspring, and reproduced pathogen-specific adaptive cellular immune responses that highly resemble the CD4+ T-cell profile observed in autistic children. In the brain, STAg-MIA induced IL-6 upregulation in astrocytes, and altered neuronal connectivity, as revealed by diffusion tensor imaging (DTI). Moreover, the MIA offspring mice manifested autism-relevant behaviors, namely abnormal social interactions, communication deficits and repetitive stereotyped behaviors.
Based on dysregulated CD4+ T-cell profile, to rescue MIA-induced immunological and behavioral abnormalities, the scientists employed adoptive cell transfer (ACT) of activated Treg cells (CD4+CD25+Foxp3+). The results showed that Treg ACT largely reversed the MIA-induced pro-inflammatory immune profile and behavioral abnormalities in these mice. Unexpectedly, rescue experiments revealed significantly augmented therapeutic efficacy by pathogen-specific maternal Treg cells. By using unbiased single-cell RNA sequencing, the researchers identified and characterized a unique group of Tregs that constitute ~32.6% of the pathogen-activated maternal Treg population that may underlie the augmented therapeutic efficacy. A subset of differentially expressed genes indicating enhanced chemotaxis and migration were verified at functional levels. The research also found that IL-10, programmed cell death protein 1 (PD1) and inducible T-cell costimulator (ICOS) were upregulated in these Treg cells, that can suppress pro-inflammatory activities.
This study developed a new preclinical parasite-mimicking MIA animal model, presented mechanistic insights into pathogenesis of MIA-induced phenotypes and demonstrated potential feasibility of managing neurodevelopmental conditions by immune cell therapy in peripheral circulation. The identification and molecular characterization of the activated maternal Treg cells by scRNA-seq may provide a blueprint for cell engineering to generate highly efficacious therapeutic Treg cells.
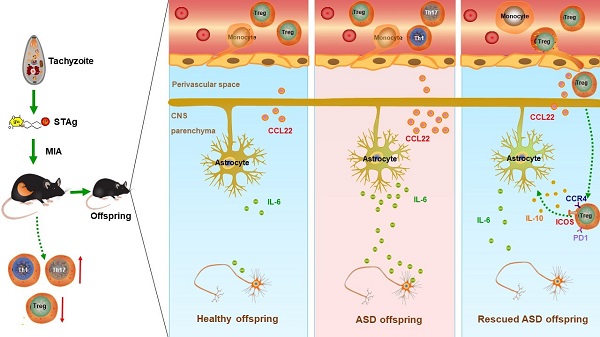
Graphical abstract: A proposed model showing STAg-MIA which induces immuno-neurological dysfunctions in adult offspring that can be reversed by adoptive transfer of Treg cells. (Image by XU Zhipeng)
Link to article: https://www.nature.com/articles/s41593-021-00837-1
Contact: diaowentong@simm.ac.cn




