Structural and Functional Studies of Adhesion Receptors Disclose Intrinsic Activation Mechanism of Orphan GPCRs
Nature study provides new clues for cancer drug discovery
G protein-coupled receptors (GPCRs) play a crucial role in cell signal transduction and comprise the largest drug target protein family. Despite recent breakthroughs in structural and pharmacological studies of these receptors, over 100 GPCRs remain orphan receptors, i.e., their ligands and signaling pathways are unknown. This limits full understanding of the physiological functions and signaling mechanisms of the GPCR superfamily. As a “virgin land” of drug discovery, orphan receptors thus offer new opportunities for drug development by serving as potential drug targets.
In a study published in Nature on April 13, a research team led by WU Beili and ZHAO Qiang of the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, in collaboration with a group led by SHUI Wenqing of ShanghaiTech University, made a breakthrough in the field of orphan receptors by determining four cryo-electron microscopy (cryo-EM) structures of two adhesion receptors, ADGRD1 and ADGRF1, in complex with G proteins and by carrying out extensive functional studies.
Adhesion GPCRs (aGPCRs), which comprise 33 receptors, participate in a variety of physiological processes such as immune responses, organ development and cellular communication. These receptors are involved in many diseases, including schizophrenia and cancers. However, the aGPCR family is by far the least understood class of GPCRs, with orphan receptors representing the majority of the family, thus hampering drug discovery.
ADGRD1 and ADGRF1 had both been recognized as oncogenes in various cancers, but their activation and modulation mechanisms were still elusive. This time, however, the researchers made exciting progress by solving the complex structures of ADGRD1 and ADGRF1 bound to G proteins. These structures reveal many unique features of receptor signal transduction and function modulation. Notably, the researchers found that a segment between the extracellular domain (ECD) and transmembrane domain (TMD) of the receptor—known as the “stalk”—acts as a tethered agonist.
The stalk activates the receptor by interacting with the receptor TMD, thus leading to conformational changes of the transmembrane helices and subsequent G protein coupling. This structural feature has not been observed in any other GPCR structures and highlights the uniqueness of the signaling mechanism of the aGPCR family.
Unlike other GPCRs, the aGPCRs have an extended N-terminal ECD that contains various adhesion domains and a well-conserved GPCR autoproteolysis-inducing (GAIN) domain. Most aGPCRs are autoproteolytically cleaved at a highly conserved GPCR proteolysis site (GPS) in the GAIN domain, thus resulting in two noncovalently associated fragments.
The segment between the GPS and TMD is the stalk. The G protein-bound structures of ADGRD1 and ADGRF1 show that the stalk dissociates from the GAIN domain and then penetrates into a binding pocket within the TMD. This finding demonstrates the importance of the conformational rearrangement of the stalk in receptor activation.
Autoproteolysis was originally believed to facilitate stalk dissociation and receptor activation. However, the proteolysis-deficient mutants of ADGRD1 and ADGRF1 showed a wild-type level of activity. More intriguingly, the researchers determined the G protein-bound structure of the proteolysis-deficient ADGRF1 and observed a stalk-TMD interaction mode similar to that in the auto-cleaved receptor. These data strongly imply that autoproteolysis is not required for receptor activation.
Despite poor sequence similarity in the TMD regions of ADGRD1 and ADGRF1, these two receptors accommodate their stalks through similar interactions, suggesting a conserved stalk-TMD binding pattern in different aGPCRs.
Further inspection of the structures revealed a cascade of interaction clusters within the receptor TMD, which relays stalk-induced conformational changes in the receptor extracellular region to the intracellular side. The importance of these interactions is supported by functional studies showing that mutations within the interaction cores substantially impair receptor signaling. These molecular details would facilitate drug development targeting these two aGPCRs.
Another important finding of this study is that a natural lipid molecule specifically binds to ADGRF1 and modulates receptor function. Using lipidomics analysis, this lipid molecule has been identified as lysophosphatidylcholine (LPC). Supported by functional data, the LPC molecule facilitates receptor activation by stabilizing the receptor in the active state. This is the first case of an LPC ligand being associated with a GPCR and is important for receptor function modulation.
This study uncovers for the first time key molecular factors that govern intrinsic activation of adhesion receptors, thus providing essential insights into the signal transduction mechanism of this GPCR family and offering new clues for drug design and discovery.
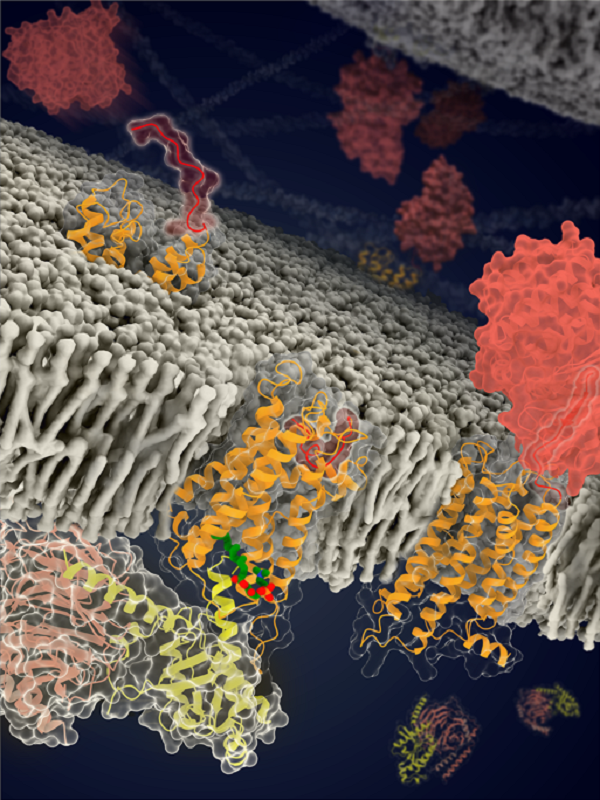
the structures and models of ADGRF1 in distinct conformational states (Image by Beili Wu’s laboratory at SIMM)
(The stalk, TMD and GAIN domain of the receptor are colored red, orange and dark red, respectively. The LPC is colored green and the three subunits of the G protein are colored yellow, light pink and light grey, respectively.)
Link to the paper: https://www.nature.com/articles/s41586-022-04580-w
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




