Researchers Reveal Major Pitfalls Plaguing Structural Elucidation of Marine Natural Products
Natural Products (NPs) have long been an important source for drug discovery. Accurate knowledge of the constitution, stereochemistry, and conformation of a bioactive natural product is critical. Developments in spectroscopic techniques make the structural elucidation of NPs more efficient. However, the flow of structural revisions that regularly appears in the literature show that attention still needs to be paid to the accuracy of structural elucidation of NPs.
Incorrect structures are especially common in marine natural products (MNPs) since the special environment of the ocean generates a more diverse chemical landscape compared with the one associated with terrestrial plants. In addition, the inaccessibility of marine organisms makes it difficult for researchers to discover and revise the misassigned structures.
In a review article published in Natural Product Reports on June 30, a team of researchers led by GUO Yue-Wei from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, in collaboration with Giovanni Appendino from Università degli Studi del Piemonte Orientale, Italy, summarized 215 cases of misassigned MNPs reported between July 2010 and August 2021 and discussed major pitfalls still plaguing the structural elucidation of small molecules, emphasizing the role of total synthesis, crystallography, as well as chemical- and biosynthetic logic to complement spectroscopic data.
The researchers summarized the structural elucidation technique mostly responsible for the structural misassignments and revisions of MNPs between 2010 and 2021, respectively. Errors in NOE-analysis (23%) and in the comparison of NMR spectra (23%) are the main culprits for structural misassignments, followed by techniques of chemical derivatization (10%) and MS analysis (7%). On the other hand, the correction of MNP misassignments is generally based on total synthesis (38%), a reinterpretation of NMR data (17%), X-ray diffraction analysis (9%). In addition, the relevance of computer-aided methods is constantly growing, and is responsible for ca. 17% of the reported corrections.
Subsequently, according to the structural elements associated with the wrong assignments, the researchers sorted out 215 cases into eight groups, including wrong carbon–carbon connectivity assignment, constitution of a heterocyclic ring scaffold, functional group misidentification, functional group mispositioning, absolute configuration, single stereocenter, multiple stereocenters, and double bond geometry. Based on a comparative analysis of the original and the revised structures, the researchers discussed in detail the logic that led to the error, and how the error was corrected.
The researchers also compared the structural misassignments of terrestrial and marine natural products, and they found that distinct “trends” in natural product misassignment are evident between compounds of marine and plant origin, with an overall much lower incidence of “impossible” structures within misassigned MNPs.
The researchers pointed out the deficiencies/pitfalls of some technologies and methods that are easy to lead to wrong conclusions, and gave suggestions for some specific issues. They concluded that “no single technique can unambiguously assign the structure of a complex unknown compound, but the synergistic combination of information from different techniques can do it with high reliability”. The recognized “gold standard” for the structural elucidation of NPs includes X-ray diffraction analysis and total synthesis.
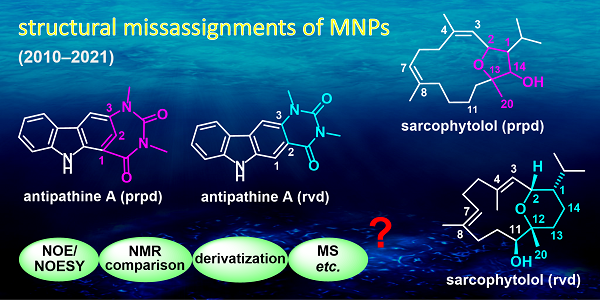
CAPTION: The major pitfalls plaguing the structural elucidation of MNPs
CREDIT: Image by GUO Yue-Wei's laboratory at SIMM
DOI: https://doi.org/10.1039/D2NP00023G
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




