Chinese Scientists Show How Fentanyl and Morphine Act on μ Opioid Receptor
Pain, especially chronic pain, is a common neurological phenomenon. The most common chronic pain conditions include low back pain, arthritis pain, migraine and cancer pain—all of which seriously affect people’s physical and mental health. Opioids, such as morphine and fentanyl, are currently the most widely used potent pain-relief drugs. They produce analgesic effects by acting on G protein-coupled receptors, the opioid receptors.
Most of the opioids used clinically are agonists of the μ opioid receptor (μOR). The use of opioid drugs causes many side effects including addiction, respiratory depression, and constipation. The analgesic effects of opioids were previously reported to be mediated through the G protein signaling pathway, while the side effects were caused by the arrestin signaling pathway of μOR. However, the lack of molecular understanding of the preferential G protein signaling mechanism of μOR has greatly hindered the rational design and discovery of G protein-biased μOR agonists for potentially safer pain treatment.
In a study recently published in Cell, research teams led by H. Eric Xu, ZHUANG Youwen, XIE Xin, and WANG Mingwei from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences reported and analyzed the high-resolution cryogenic electron microscopy (cryo-EM) structures of human μOR activated by opioid analgesics such as fentanyl, morphine, and oliceridine, thus revealing for the first time the mechanisms of recognition and activation of μOR induced by fentanyl and morphine.
The researchers first obtained the three-dimensional structures of human μOR bound to balanced agonists such as fentanyl, morphine, and peptidomimetic Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol (DAMGO). These agonists exhibited both G protein and arrestin signaling activities. The researchers also solved μOR complexed with G protein-biased agonists such as TRV130, SR17018, and PZM21.
Then they analyzed the signaling properties of μOR under the activation of different signaling agonists by functional analysis at the cellular level and molecular dynamics simulations. The results showed that, compared with morphine, fentanyl occupied an extra binding pocket at the extracellular side of μOR around TM2 and TM3. In addition, the aniline ring side chain of fentanyl formed π-π hydrophobic interactions with residues W295 and Y328. The more extended interactions of fentanyl with μOR contributes to the 50-100 times higher potency of fentanyl compared with morphine.
Based on the structure of fentanyl-bound μOR, the researchers further explored the structure-activity relationship (SAR) of fentanyl and its derivatives with μOR using molecular docking and mutagenesis studies. They found that the potencies of fentanyl and fentanyl analogs were highly associated with varying degrees of interaction of the ligands with μOR residues such as D149, Y150, W135, and W320.
Intensive structural analysis and molecular dynamics simulations revealed that G protein-biased agonists such as PZM21 tend to bind to the TM2/3 side of the μOR ligand-binding pocket. In contrast, balanced agonists like fentanyl and DAMGO showed more extensive and balanced interactions with μOR transmembrane domains, leading to μOR having a more compact intracellular cavity. This condition was favorable for the arrestin coupling of μOR, thus explaining the molecular determinants necessary for the arrestin activity of μOR.
Fentanyl and its analogs constitute the major cause of the “opioid crisis.” However, how they bind and activate μOR has remained elusive. This study presents the structure of fentanyl-bound μOR for the first time and reveals the specific fentanyl-binding mode compared with that of morphine. It provides insight into the SAR of fentanyl and its analogs. It also increases molecular understanding of the biased agonism and ligand selectivity of μOR through the combined use of multiple functional assays and molecular dynamics simulation. This study deepens understanding of the regulation mechanism underlying μOR signal transduction and may facilitate development of next-generation opioid analgesics with fewer side effects.
Web link:
DOI:
https://doi.org/10.1016/j.cell.2022.09.041
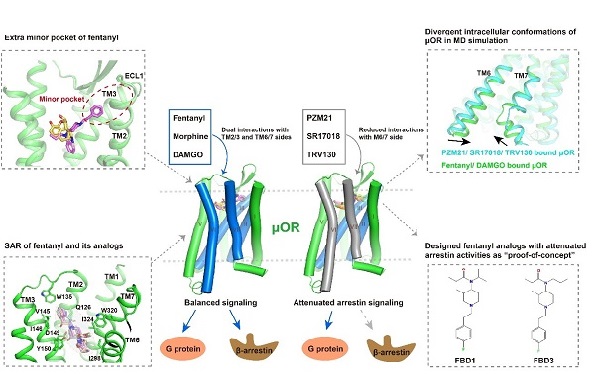
Figure 1. Structures of human μOR bound to opioids with different chemical scaffolds. Top left: Different binding modes of fentanyl and morphine; Bottom left: Structure-activity analysis of the interaction of fentanyl and its derivatives with μOR; Top right: Balanced agonists of μOR compared to G proteins in molecular dynamics simulations. Balanced agonists mediate a more constricted intracellular conformation. Bottom right: novel G-protein-biased fentanyl derivatives FBD1 and FBD3 with different potencies. Middle: Attenuating the interaction of ligands and TM6/7 of μOR causes G protein signaling bias.
CONTACT:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn 



