Cytokine IL6 May Support Long-term Expansion of Hepatocytes in vitro
Liver is the primary metabolic organ in mammals. During homeostasis, the liver cells generally have a low turnover rate. However, liver exhibits robust regeneration following chemical, viral or mechanical injuries, attributing to the immediate proliferation of hepatocytes. After 2/3 partial hepatectomy (PHX) in mice, it takes only 1 week for the remaining liver to regenerate to the original size. Comparing to the robust proliferation capacity in vivo, adult hepatocytes have been proven to be extremely difficult to culture and expand without losing their hepatic characteristics in vitro. A few studies have demonstrated that chemical cocktails with growth factors or Wnt ligands can support long-term expansion of hepatocytes via dedifferentiation. However, the culture conditions are complex, and clonal expansion of hepatic progenitors with full differentiation capacity are rarely reported.
Due to the wide application of primary hepatocytes in drug toxicity and metabolism evaluation, and the potential use of hepatocyte cell therapy in liver diseases, to identify ways to expand hepatocytes in vitro is an urgent task. In a recent study published in Nature Communications, a team of researchers led by XIE Xin from Shanghai Institute of Materia Medica of Chinese Academy of Sciences, in collaboration with researchers from Shanghai University of Science and Technology, Fudan University and Hangzhou Institute for Advanced Study of UCAS, reported a physiological and simple way to culture hepatocytes.

In this research, the scientists discovered that IL6, a cytokine which has been reported to play a critical role in liver regeneration in vivo, could promote a long-term, almost indefinite expansion of primary mouse hepatocyets in vitro in simple 2D culture, in combination with EGF and HGF (passaged >50 times at publication), with the theoretical expansion of the cells at 30 passages and ~150 days reaches ~1035 times. The IL6-medium supported the growth of primary hepatocytes by dedifferentiating the cells into induced hepatic progenitor cells (iHPCs), which maintain the capacity of differentiation into functionally mature hepatocytes. IL6-medium also supported the establishment of single hepatocyte-derived iHPC clones. Hepatocytes (iMHs) differentiated from these iHPCs could easily engraft and repopulate the whole liver of Fah-/- mice in 2 monthes.
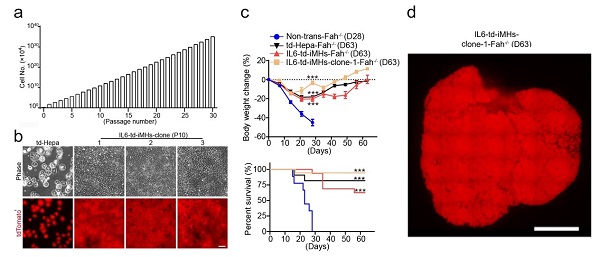
Figure 1. a, The expansion of iHPCs in IL6-medium. b, Establishment of single hepatocyte-derived iPSC clones. c, IL6-iMHs transplantation saves Fah-/- mice. d, Repopulation of the live of Fah-/- mouse after IL6-iMHs transplantation.
Diploid and polyploid hepatocytes are all cound in mature liver. The exact functional differences between these cells is still unclear. In this study, IL6-medium has been found to mainly promote the proliferation of diploidy hepatocytes. IL6-medium mainly activated the STAT3, ERK and AKT pathways. By systemically comparing the global gene expression profiles in regenerating livers and hepatocytes culturing in various conditions, a number of transcription factors including Barx2, FoxM1, Elf3 and Mxd3 have been identified to be downstream of STAT3, ERK and AKT pathways and to regulate hepatocytes dedifferentiation and growth. This physiological and simple way may provide ideas for culturing previously difficult-to-culture cell types and support their future applications.
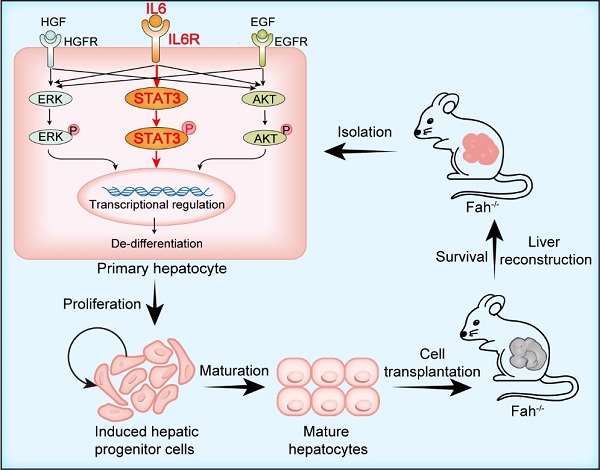
Figure 2. IL6-medium stimulates hepatocyte dedifferentiation and growth by activating STAT3, ERK and AKT pathways and the downstream transcription factors
CONTACT:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




