Motilin is a peptide consisting of 22 amino acids that is secreted by cells in the gastrointestinal (GI) tract. It exclusively activates the motilin receptor (MTLR), a G protein-coupled receptor (GPCR), which is specifically expressed in endocrine organs and the GI tract. The MTLR-motilin signaling pathway is indispensable for regulating human GI motility, hormone secretion, and feeding/hunger signals.
The motilin receptor (MTLR) and the ghrelin receptor (GHSR) share a high degree of sequence homology, with an 86% identity in their transmembrane domains. The peptide hormone motilin also shares 36% sequence homology with ghrelin. Despite these similarities, motilin and ghrelin exhibit high binding selectivity for MTLR and GHSR with unknown mechanisms.
MTLR can also be activated by classical macrolide antibiotics such as erythromycin. Besides its anti-microbial effect by inhibiting bacterial ribosomal assembly, erythromycin also leads to GI side effects such as diarrhea, nausea, and vomiting. In the 1980s, scientists discovered that erythromycin could activate MTLR to elicit GI responses, marking MTLR the first identified GPCR that could be activated by an antibiotic. So far, the recognition pattern of MTLR by erythromycin remains elusive.
In a study published in Science Advances, a team of researchers led by H. Eric XU (XU Huaqiang) from Shanghai Institute of Materia Medica of the Chinese Academy of Sciences and JIANG Yi from LinGang Laboratory resolved two high-resolution cryo-EM structures of Gq-coupled MTLR bound to motilin and erythromycin, respectively. These structures clarify the unique motilin-binding mode of MTLR and reveal the peptide selectivity mechanisms of both MTLR and GSHR. Furthermore, the researchers have identified the novel recognition mode of erythromycin by MTLR and elucidated the similarities and differences of erythromycin recognition between MTLR and the bacterial ribosome. These findings deepen our understanding of the mechanism underlying peptide selectivity and the GI side effects of erythromycin. These structures also offer a new opportunity for designing GI motility and anti-bacterial drugs with fewer GI side effects.
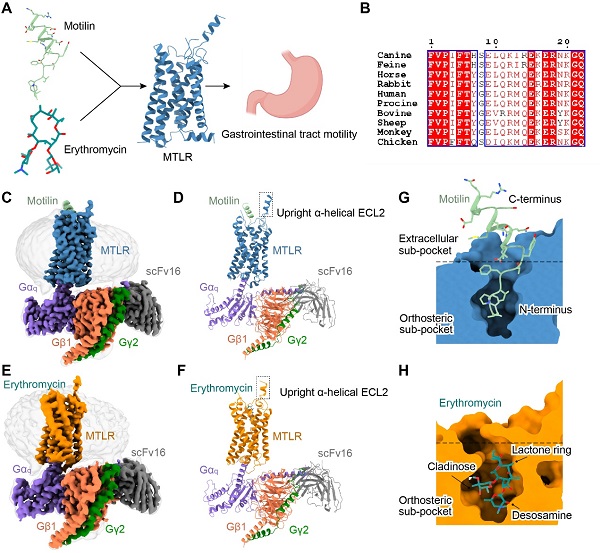
Figure. 1. Overall structures of Gq-coupled MTLR complexes bound to motilin and erythromycin. (A) Schematic illustration of ligands of MTLR and physiological effects of MTLR. (B) Sequence alignment of motilin across different species. (C-F) Orthogonal views of the density maps and models of motilin-MTLR-Gq-scFv16 (C, D) and erythhromycin-MTLR-Gq-scFv16 complexes (E, F). (G-H) Cut-away view of binding sub-pockets of MTLR for motilin (G) and erythromycin (H).
Contact :
DIAO Wentong
Shanghai Institute of Materia Medica
Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




