Groundbreaking Research Unveils the Structural Basis for Polypharmacology in Dopamine Receptor System
Neurotransmitter dopamine and its receptors play pivotal roles in the central nervous system in humans, regulating various physiological functions such as motor control, cognitive abilities, emotional regulation, and reward mechanisms. Abnormal dopamine signaling is linked to numerous neuropsychiatric disorders, including Parkinson's disease, schizophrenia, depression, and addiction. Polypharmacology of drug molecules, a concept referring to a single drug's ability to interact with multiple receptor targets, is a critical area of study. The exploration of polypharmacology of drug molecules has long been a focus and challenge in the field of GPCR pharmacology due to its far-reaching implications on drug efficiency and safety. Drugs targeting dopamine receptors for neurodegenerative diseases like Parkinson's disease and schizophrenia often exhibit polypharmacological characteristics. However, research on their working mechanisms is limited. By studying the structural pharmacology of dopamine receptors and elucidating the molecular mechanisms behind the selective interaction of drug molecules, we can better understand the activation of the dopamine receptor system. This understanding also aids in the design of effective dopamine receptor-targeted drugs with reduced side effects. This research is integral to the field as polypharmacology opens new avenues for the development of novel therapeutic interventions and helps overcome long-standing scientific challenges.
Dopamine receptors belong to the G protein-coupled receptor (GPCR) superfamily, which includes five receptor members (D1R to D5R). Over the past decade, international research teams have made significant progress in the field of dopamine receptor structural biology, greatly improving our understanding of the system's mechanisms and promoting the development of drugs targeting the dopamine system. However, the structure of D5R and the activated state of D4R remain elusive, limiting our understanding of ligand recognition and activation mechanisms of the dopamine receptor family, thereby hindering the development of structure-based drugs targeting dopamine receptors.
On May 23th 2023, in a study published in the Cell Research, a team of researchers led by XU Huaqiang (H. Eric XU) from Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences, Bryan Roth from the University of North Carolina at Chapel Hill, and ZHANG Yan from Zhejiang University jointly revealed the structure of dopamine receptor D5R and activated dopamine receptor D4R, and systematically analyzed the polypharmacology and signaling mechanisms of the entire dopamine receptor system.
The team employed single-particle cryo-electron microscopy to resolve the structures of all five dopamine receptors bound to the same agonist molecule, Rotigotine, as well as downstream G protein complexes.
Although all five dopamine receptors exert their physiological functions by binding to the same endogenous ligand dopamine, subtle structural differences between the receptors can affect their affinity with drug molecules and activate entirely different signaling effects. The researchers compared the structures of the five receptors and found obvious differences between D1-like and D2-like receptors. Rotigotine, a drug used to treat Parkinson's disease and restless leg syndrome, can activate all five dopamine receptors and is a typical polypharmacological molecule. The researchers systematically compared the binding modes of Rotigotine with different dopamine receptors and validated the interactions between ligands and receptors through pharmacological experiments.
In their quest to understand the correlation between different receptors' ligand-binding pockets and the polypharmacological traits of small molecules, the research team undertook a screening of Rotigotine's binding activity against more than 300 GPCRs. They discovered that Rotigotine's affinity extends beyond dopamine receptors to a variety of other GPCRs, including serotonin receptors, adrenergic receptors, somatostatin receptors, adenosine receptors, and opioid receptors. By comparing structures and sequences, the researchers identified a direct link between a ligand's ability to broadly bind and the conservativeness of orthosteric binding pocket.
Additionally, the study revealed the effect of cholesterol on the function of dopamine receptors. The researchers found a cholesterol molecule near the second intracellular loop of D5R stabilizing the conformation of a residue Tryptophan, which contribute to the selectivity of positive allosteric modulators bind to D1R. A cholesterol molecule was also found between the first and seventh transmembrane helices of D4R, indirectly affecting ligand-receptor binding.
Additionally, the study highlighted the impact of cholesterol on dopamine receptor functionality. Researchers found a cholesterol molecule close to the second intracellular loop of D5R, stabilizing the conformation of a Tryptophan residue. This stabilizing action inhibits the binding of D1R-selective positive allosteric modulators to D5R, thereby directing the selective binding to D1R. Furthermore, a cholesterol molecule situated between the first and seventh transmembrane helices of D4R was found to indirectly influence the ligand-receptor binding process.
This extensive structural study of dopamine receptors underscores the team's commitment to refining our understanding of polypharmacology, paving the way for more efficient and safer drug development in the future.
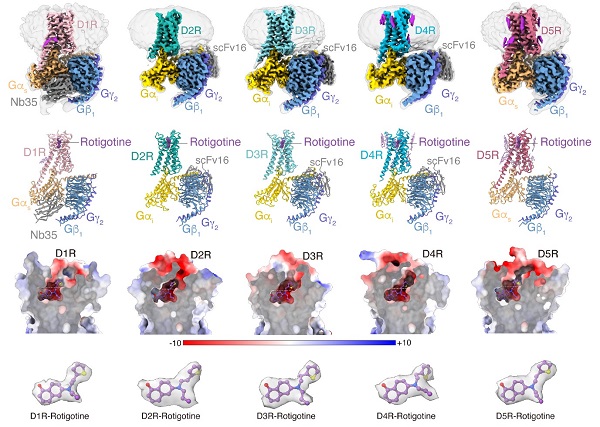
Cryo-EM structure of five dopamine receptors and binding patterns of rotigotine to dopamine receptors (Image by XU Peiyu)
DOI: https://doi.org/10.1038/s41422-023-00808-0
LINK: https://www.nature.com/articles/s41422-023-00808-0
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




