Researchers Reveal Recognition of Medium-Chain Fatty Acids by GPR84
Free fatty acids are hydrocarbon chains terminated by carboxylic acids. They not only serve as an important source of energy for humans and animals, but also act as signaling molecules in cellular functions. Medium-chain fatty acids (MCFAs), such as capric acid (C10), undecylic acid (C11), lauric acid (C12) and their hydroxy forms (e.g. 3-OH-C12), have chain lengths of 8 to 12 carbons, which play an important role in energy metabolism and inflammation. Disruption of the beta-oxidation of fatty acids, the process associated with 3-OH-C12 production, can result in increased plasma concentrations of 3-OH-C12 to micro molar levels. In addition, researches have shown that 3-OH-C12 can modulate the release of the pro-inflammatory cytokine IL-6, highlighting the important role of MCFAs in inflammation.
GPR84, a member of the class A G protein-coupled receptors (GPCRs), has been the subject of debate as to whether medium-chain fatty acids, including 3-OH-C12, act as its endogenous ligands due to their relatively low potency. And the high expression of the receptor in immune cells such as peripheral monocytes, macrophages, neutrophils and microglial cells in the brain has confirmed its role as an inflammatory receptor and a potential therapeutic target for several inflammatory diseases, including ulcerative colitis, fibrotic diseases, non-alcoholic fatty liver disease and acute respiratory distress syndrome.
In previous studies, a team of researchers led by XIE Xin and NAN Fajun developed a new GPR84-selective antagonist named as BGT-004 (compound 33), which showed remarkable efficacy in reducing intestinal inflammation in a mouse model of inflammatory bowel disease, and outperformed the clinical drug mesalazine. In addition, the team got a GPR84 agonist, ZQ-16, through a large-scale screening of the National Compound Library. Subsequent optimization efforts led to the discovery of LY-237, the most potent GPR84 agonist known to date.
In a study published in Nature Communications in June, researchers led by Xu Huaqiang(H. Eric XU), Xie Xin and Yin Wanchao from Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, used cryo-electron microscopy (cryo-EM) to elucidate the structure of the GPR84-Gαi complex activated by the LY237 at a resolution of 3.23 angstrom (Fig1a). Surprisingly, without exogenous agonists supplied, the researchers resolved a structure of the GPR84-Gαi complex with a small molecule density observed in the orthosteric binding site of GPR84 (Fig1b). Inspired by analysis of the small molecule density feature and functional experiments, the researchers further determined the structure of the GPR84-Gαi complex in the presence of 3-OH-C12 at a resolution of 2.89 angstrom. The similar densities between these two structures provided preliminary evidence for 3-OH-C12 as an endogenous ligand of GPR84 (Fig1c-d).
The study revealed several unique structural features of GPR84. The extended C-terminus of transmembrane helix 5 (TM5) was found to have extensive interactions with the downstream Gαi protein. The extracellular loop 2 (ECL2) formed a combination of two disulfide bonds with TM3 and the N-terminus, leading to the temporary closure of the ligand binding pocket from the extracellular milieu(Fig1e-f). The hydrophobic amino acid cluster between TM4/5 formed a barrier that precisely accommodated the alkyl tails of LY237 and 3-OH-C12, allowing accurate ligand selectivity based on the alkyl chain lengths. In addition, the positively charged side chain of R172 on the ECL2 played a critical role in anchoring and stabilizing the polar end of the agonist molecule LY237 or 3-OH-C12.
The researchers also investigated the pathway for ligand entry into the binding pocket of GPR84. Molecular dynamics simulations and mutational functional experiments indicated that in the initial stage of agonist ligand recognition, polar amino acids on the extracellular side of GPR84, in particular R349 and H352, attract the negatively charged head of the ligand, leading to conformational changes in the extracellular side of the GPR84 that facilitate the entry of the agonist into the binding pocket. This highlighted the critical role of ECL2 not only in directly participating in agonist binding, but also in the process of ligand entry from the extracellular environment.
In addition, the study revealed a unique 'dual toggle switch' mode of the GPR84 activation. The conserved residue Y332 at the position 6.48 interacts with the corresponding residue N104 at the position 3.36 through hydrogen bonding upon agonist binding, facilitating coordinated conformational changes in the intracellular side of the receptor, including the DRY and NPxxY motifs, leading to Gαi protein coupling. These findings provide a deeper understanding of the diverse activation mechanisms of class A GPCRs.
The research not only sheds light on the ligand recognition and entry process of GPR84, but also provides valuable insights into the physiological functions of GPR84 and its potential as a therapeutic target. These findings may pave the way for further studies of GPR84 in various inflammatory diseases and the development of novel drugs targeting GPR84.
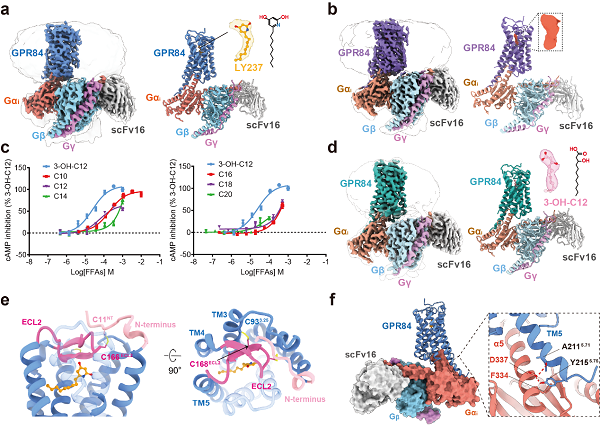
cryo-electron microscopy structures of the GPR84-Gαi protein complex (Image by XU Huaqiang’s laboratory of SIMM)
Link: https://www.nature.com/articles/s41467-023-38985-6
DOI: https://doi.org/10.1038/s41467-023-38985-6
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: diaowentong@simm.ac.cn




