Researchers Reveal New Mechanism of Physical Pharmacy for Drug Delivery
Nanoparticles for drug delivery play a crucial role in regulating the spatial and temporal distribution of active pharmaceutical ingredients (including small molecules, peptides, proteins, and nucleic acids) within the body, thereby improving drug safety and efficacy. However, these nanoparticles face numerous physiological and pathological barriers in vivo that severely limit their delivery efficiency. Understanding interactions between nanoparticles and biological barriers is essential for overcoming these challenges. Recent studies have demonstrated the importance of physical properties of nanoparticles, such as shape, stiffness, size, and surface characteristics, in determining their interactions with these complex barriers.
In a study published in the Proceedings of the National Academy of Sciences (PNAS) in May 2024, a research team led by GAN Yong from Shanghai Institute of Materia Medica (SIMM) of Chinese Academy of Sciences (CAS), in collaboration with SHI Xinghua from National Center for Nanoscience and Technology (NCNST) of CAS, unveiled that nanoparticles with high curvature, specifically fusiform-shaped nanoparticles, facilitate rapid extravasation and penetration against interstitial fluid pressure (IFP), significantly enhancing drug delivery efficiency.
In this study, researchers designed and synthesized nanoparticles with different curvatures while keeping their hydrodynamic diameters, surface chemistries, and zeta potentials consistent. Detailed characterization using transmission electron microscopy (TEM) revealed that fusiform-shaped nanoparticles with the highest curvature (ENP5) had superior extravasation and penetration abilities in high IFP environments compared to the spherical, ellipsoidal, and rod-shaped nanoparticles. In mouse models, ENP5 loaded with the chemotherapy drug doxorubicin (Dox) showed significantly enhanced tumor suppression, achieving over 90% inhibition and extending the survival of mice with liver cancer.
Using high-resolution microscopy and molecular simulation techniques, the team explored how these nanoparticles move and function in high IFP environments. They found that the high curvature of fusiform-shaped nanoparticles reduces fluid resistance, speeding up their migration during extravasation. Once inside the tissue, their curvature promotes rotational motion, increasing their movement through the dense extracellular matrix, thus improving drug delivery efficiency.
This research emphasizes the importance of nanoparticle physical properties in enhancing delivery efficiency. It highlights innovative use of curvature as a precise quantitative descriptor of nanoparticle shape, significantly improving characterization beyond traditional categories like rods and spheres. By focusing on curvature, the study underscores its critical role in efficient transport in high-IFP environments, bridging the gap between biology and physics. This research offers new insights into nanoparticle design for improved drug delivery in challenging environments like tumors, advancing cancer treatment and other therapeutic applications by overcoming elevated interstitial fluid pressure.
GAN’s team has been dedicated to study of physical pharmaceutics for efficient nanoparticle delivery for years. They previously identified rules and microscopic mechanisms by which physical properties such as shape, rigidity, and surface characteristics influence nanoparticle transport efficiency and target site release. These studies were published in prominent international journals, including Nature Communications (2018, 2022, 2024), Science Advances (2020), PNAS (2019), and Journal of Extracellular Vesicles (2022). These findings offer new perspectives for rational design of efficient nanoparticles to navigate complex physiological barriers.
DOI: 10.1073/pnas.2319880121
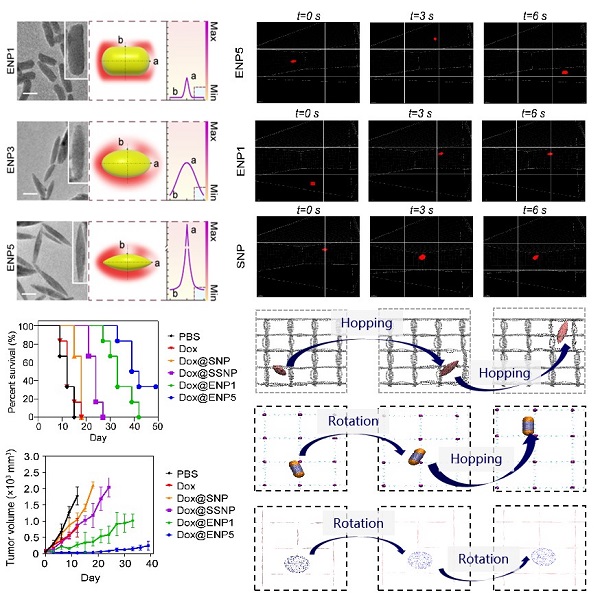
Curvature-mediated efficacy and microscopic mechanisms of nanoparticle transport (Image by GAN YONG's Laboratory)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




