Researchers Unveil Molecular Mechanisms of Somatostatin Receptor 5 Activation by Neuropeptides and Drugs
Somatostatin receptors (SSTRs) constitute a crucial family of G protein-coupled receptors (GPCRs) that play pivotal roles in regulating hormone secretion and inhibiting tumor growth. Among five subtypes (SSTR1-SSTR5), SSTR5 stands out as a highly expressed receptor in the pituitary gland, governing the release of hormones such as adrenocorticotropic hormone, prolactin, and growth hormone. Consequently, SSTR5 emerges as a promising therapeutic target for treating endocrine disorders and tumors associated with hormonal imbalances.
In a study published in Proceedings of the National Academy of Sciences (PNAS) on June 18, 2024, a research team led by Eric H. Xu (XU Huaqiang) and ZHAO Lihua from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, employed single-particle cryo-electron microscopy techniques to unveil three-dimensional structures of SSTR5 in complex with the natural neuropeptide agonist cortistatin-17 (CST17) and clinically approved drug octreotide. This remarkable achievement shed light on molecular mechanisms underlying SSTR5 activation by these ligands, paving the way for the development of more effective and selective therapeutics.
In this study, researchers meticulously determined cryo-EM structures of SSTR5 bound to CST17 and octreotide at resolutions of 2.7 angstrom and 2.9 angstrom, respectively. These findings revealed that the binding of these agonists triggers a rearrangement of a "hydrophobic lock" formed by the transmembrane helices TM3 and TM6, leading to an outward movement of TM6. This conformational change facilitates the coupling and activation of G protein, initiating downstream signaling cascades. Remarkably, structural and functional analyses unveiled distinct recognition modes of extracellular loops ECL2 and ECL3 for CST17 and octreotide, providing insights into their agonist selectivity and specificity.
The significance of this study lies in its elucidation of the activation mechanisms of SSTR5 and its selective recognition of neuropeptide and drug agonists. These groundbreaking findings pave the way for structure-based design of novel, highly selective SSTR5 modulators with reduced off-target effects, offering promising therapeutic opportunities for treating a wide range of conditions, including acromegaly, pituitary adenomas, neuroendocrine tumors, and hormonal imbalances. By harnessing the power of structural biology and rational drug design, researchers can now explore more effective and targeted interventions for these debilitating disorders.
DOI: 10.1073/pnas.2321710121
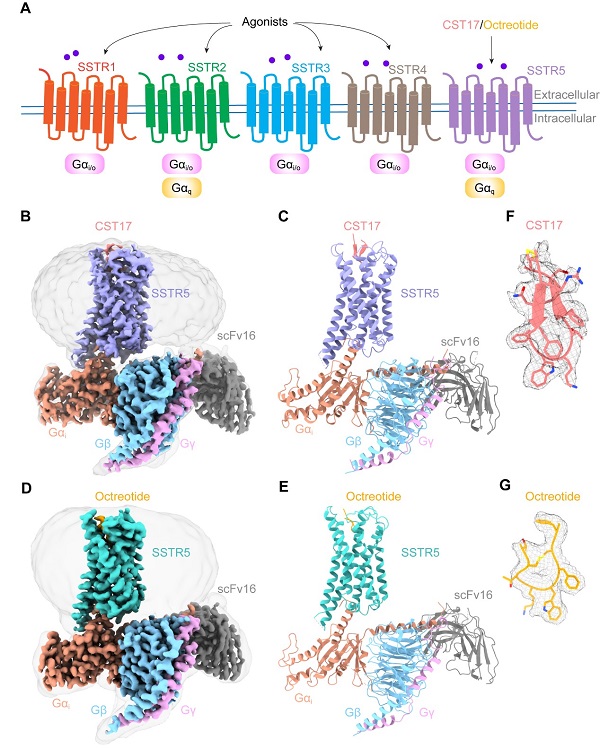
Cryo-EM structures of the SSTR5-Gi complexes bound to the neuropeptide agonist CST17 and the drug octreotide, respectively. (Image by LI Jingru).
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




