Prebiotic Carrier Assists Regorafenib in Treating Colorectal Cancer
Colorectal cancer (CRC) is one of the most diagnosed and mortal cancers worldwide. Regorafenib (REG), a small-molecule multi-pathway protein kinase inhibitor, was approved by the FDA in 2017 to treat metastatic CRC. Due to the poor water solubility and low oral absorption efficiency of REG, high doses and frequent administration are required to maintain effective intratumoral drug concentrations, which can lead to dose-related toxicity. Therefore, it is important to develop a drug delivery system that improves the pharmacokinetic properties and tumor targeting of REG.
Gut microbiota has been shown to affect the development of CRC through various mechanisms. Both regulating gut microbiota and improving intestinal environment can serve as complementary approaches to enhance CRC therapy. As a typical kind of prebiotic material, inulin promotes the proliferation of beneficial bacteria, regulates metabolism, maintains immune balance, and protects the intestinal barrier. Moreover, inulin is resistant to gastric acid and digestive enzymes, while is only degraded by microorganisms in the colon. Based on these properties, inulin has the potential to be a carrier material for colon-targeting oral drug delivery.
A research team from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences developed an oral molecular targeted drug delivery system based on an inulin derivative, which combined gut microbiota modulation with precise drug delivery and improved CRC therapy. This study was published in Advanced Functional Materials on September 2, 2024. Master students ZHU Runqi and YUAN Wenhui were co-first authors, and LI Yaping and YIN Qi were co-corresponding authors.
Researchers synthesized an amphiphilic inulin-ursodeoxycholic acid conjugate that self-assembled in water and encapsulated REG to form the prebiotic nanoparticle, named UIRN. UIRN remained stable in normal physiological conditions and upper gastrointestinal tract environments. An increased rate of REG release from UIRN was achieved in medium containing gut microbiota. After oral administration, UIRN delayed the adhesion-uptake-transport process of REG in the small intestine, increased REG distribution in the large intestine, prolonged drug circulation in blood, and increased intratumoral accumulation.
Meanwhile, UIRN significantly improved the composition of gut microbiota, increasing the proportion of beneficial bacteria and decreasing that of pathogenic bacteria. Also, the content of intestinal beneficial metabolites short-chain fatty acids were increased by UIRN. In the CT26 CRC mouse model, UIRN increased the intratumoral number of CD8+ T cells, induced polarization of tumor-associated macrophages towards the M1-type, promoted the maturation of dendritic cells in the lymph nodes, and reduced the number of regulatory T cells, thus enhancing the anti-tumor immune response. The tumor inhibition rate reached 95.4% during UIRN administration, and combining UIRN with oxaliplatin or immune checkpoint antibodies further prolonged survival. UIRN also exhibited functions of microbial regulation and tumor suppression in the drug-induced colitis-associated colorectal cancer mouse model.
This inulin derivative-based delivery system, integrating gut microbiota regulation and molecular targeted therapy, offered a promising strategy for CRC treatment.
DOI: 10.1002/adfm.202407685
Link: https://doi.org/10.1002/adfm.202407685
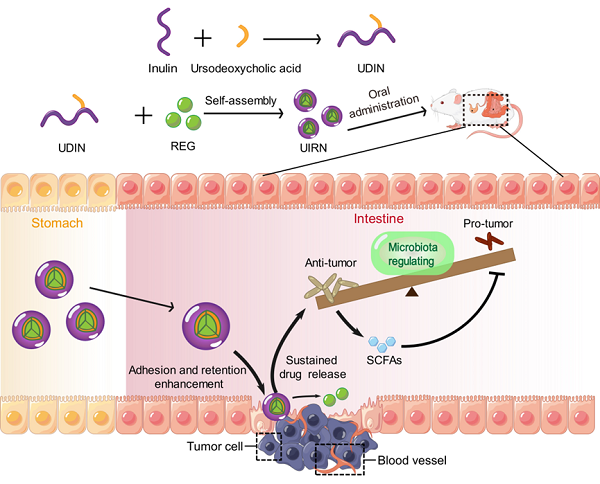
Integration of gut microbiota regulation and molecular targeted therapy by UIRN for CRC therapy (Image by ZHU Runqi)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




