Researchers Uncover Assembly and Architecture of Endogenous NMDA Receptors in Adult Cerebral Cortex and Hippocampus
Learning and memory are fundamental brain functions that underlie human cognition and perception of the world, which rely on development- and activity-dependent synaptic plasticity. N-methyl-ᴅ-aspartate (NMDA) receptors, members of excitatory ionotropic glutamate receptor family, are essential to these processes. They are characterized by distinctive biophysical properties, including high calcium permeability and voltage-dependent magnesium block. These features allow NMDA receptors to regulate strength of synaptic connections based on neuronal activity and to mediate formation of new synapses through downstream calcium signals. Therefore, they are often described as key "molecular switches" for synaptic plasticity, playing a critical role in advanced brain functions like learning and memory. In higher brain structures involved in cognition, such as the cerebral cortex and hippocampus, NMDA receptors are especially vital for cognitive function.
NMDA receptors are heteromeric tetramers composed of two obligatory GluN1 and two alternative subunits, either GluN2 (N2A to N2D) or GluN3 (N3A and N3B). Over the past decade, molecular understanding of NMDA receptors has been limited to studies conducted in recombinant over-expression systems. This is largely due to the low abundance of endogenous NMDA receptors (eNMDARs) in brain and the lack of effective purification methods, which have hindered physiological investigations of these receptors and their subtype diversity.
In a study published in Cell on January 23, a research team led by ZHU Shujia from the Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, along with LI Yang from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, dissected assembly and architecture of endogenous NMDA receptors (eNMDARs) in adult mammalian cerebral cortex and hippocampus.
The researchers enriched eNMDARs from brain tissue of adult wild-type rats using a high-affinity antibody tagged with an affinity tag. During cryo-electron microscopy (cryo-EM) data processing, they further took advantage of a convolutional network-based model to separate eNMDARs from the heterogeneous pool of endogenous proteins. By combining biochemical and algorithmic purification techniques, the researchers finally resolved the native receptors mediating physiological synaptic plasticity in the brain at near-atomic resolution. Their analysis identified three major eNMDAR subtypes: GluN1-N2A-N2B tri-heteromeric, GluN1-N2B and GluN1-N2A di-heteromeric receptors. Furthermore, they determined these three subtypes accounting for 45%, 35% and 20% of NMDA receptors in cortex and hippocampus, respectively.
The most abundant receptor subtype, GluN1-N2A-N2B tri-heteromeric tetramer, highlighted functional integration of GluN2A and GluN2B subunits in vivo. Its structure revealed a distinct assembly and asymmetric architecture. Additionally, the researchers identified conformational variations in GluN2B subunit between GluN1-N2A-N2B tri-heteromeric and GluN1-N2B di-heteromeric receptors. These structural discrepancies of the subunit across different receptor subtypes provided molecular insights into functional diversities of eNMDARs.
These findings uncovered the molecular basis by which eNMDARs precisely tune excitatory synaptic transmission and synaptic plasticity in adult mammals. Notably, subunits composition of eNMDARs undergoes alterations across different developmental stages and brain regions. The researchers made a significant advance by establishing a paradigm for mapping spatiotemporal atlas of eNMDARs throughout brain. This framework will enhance our fundamental understandings of learning and memory, such as how synaptic plasticity differs across ages. Clinically, these insights provided valuable insights into pharmacological effects of drugs targeting NMDA receptors, including ketamine, dextromethorphan and memantine. In addition, this study paved the way for exploring pathological changes in eNMDARs under different disease models.
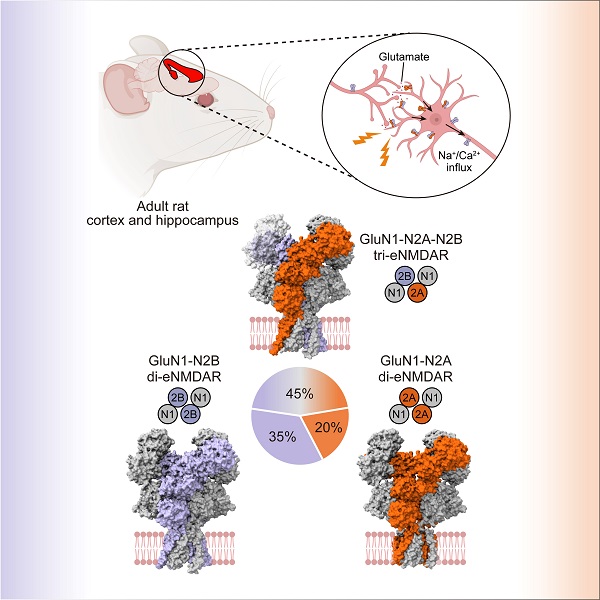
The image depicts the subunit assembly of eNMDARs isolated from the adult rat cortex and hippocampus. (Image by ZHANG Ming)
Contact:
JIANG Qingling
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
E-mail: qljiang@stimes.cn




