Chemerin Receptor Structures Disclose New Lipid Metabolism Mechanism
Chemerin is a key adipokine—a signaling protein released by adipose tissue—that regulates lipid metabolism and insulin sensitivity and thus is involved in many metabolic diseases such as obesity and diabetes. It also plays a critical role in inflammation as a chemoattractant adipokine—a molecule that guides immune cells to inflammatory sites.
Chemerin’s functions are mediated by two receptors, CMKLR1 and GPR1. CMKLR1 is a canonical G protein-coupled receptor (GPCR) that activates G proteins and promotes β-arrestin recruitment. In contrast, GPR1 is an atypical GPCR that undergoes β-arrestin internalization with weak G protein signaling. It was previously unclear how these two receptors cooperated to govern chemerin functions.
In a study published in Science on November 20, a team led by WU Beili from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, along with collaborators, determined the structures of GPR1 bound to chemerin and β-arrestin 1 or β-arrestin 2, as well as the structure of GPR1–β-arrestin 1 complex in the absence of chemerin. They elucidated a picture of arrestin-mediated modulation of GPR1, deepening our understanding of non-canonical GPCR signaling.
It has been proposed that GPCRs recruit arrestins in a multi-step process with distinct interaction patterns at different stages. However, the molecular mechanism underlying the dynamics has been poorly understood. In this study, the researchers observed that GPR1 coupled to β-arrestin 1 through at least four distinct binding modes, which reflects a dynamic transition from a “pre-coupled” state to a fully “engaged” state.
β-arrestin 1 and β-arrestin 2 have comparable binding affinities to GPR1, but the two arrestins exhibit different signaling patterns with this receptor. The researchers found that, unlike β-arrestin 1, β-arrestin 2 engages GPR1 predominantly in a single binding configuration that promotes receptor internalization and downstream signaling. This conformational difference may provide a molecular basis for differential properties of these two arrestins in defining the signaling pattern of GPR1.
Moreover, the researchers revealed that cholesterol, an important component of the cell membrane, is essential for the engagement between GPR1 and β-arrestin 2, but not β-arrestin 1, which is another difference between the binding modes of the two arrestins. This finding extends our knowledge about arrestin-mediated regulation of GPR1 and offers new clues for discovering drugs targeting specific arrestin pathways.
Numerous GPCRs including GPR1 not only promote agonist-stimulated internalization, but also undergo constitutive internalization in the absence of an agonist, which further modulates receptor functions. This is a key mechanism for GPR1 scavenging chemerin isoforms with distinct activity (agonistic or antagonistic), but the underlying mechanism has been unknown.
By solving the ligand-free structure of the GPR1–βarr1 complex, the researchers provided molecular details of constitutive engagement between a GPCR and arrestin. GPR1 adopts an inactive conformation that enables β-arrestin 1 to bind the receptor in a new interaction pattern. Mass spectrometry analysis showed that the C-terminal region of the inactive GPR1 exhibits a high basal phosphorylation level, which promotes arrestin recruitment and internalization in the agonist-free state.
Furthermore, the researchers found that endogenous fatty acids palmitoleic acid and palmitic acid facilitate the binding of inactive GPR1 to β-arrestin 1 and enhance constitutive arrestin recruitment. This suggests that these lipid molecules play a regulatory role in the scavenging of antagonistic chemerin isoforms by GPR1.
Analysis of lipid accumulation in adipocytes showed that under high-fat conditions, CMKLR1 stimulates lipid metabolism and reduces lipid accumulation, whereas GPR1 facilitates CMKLR1 activation by scavenging antagonistic chemerin, further promoting lipid metabolism.
This work provides the first molecular characterization of GPR1 as a scavenger receptor that fine-tunes chemerin signaling through arrestin-biased signaling. It highlights the complexity and diversity of molecular mechanisms underlying the arrestin-mediated modulation of GPCRs, and it offers new opportunities for developing novel therapeutic strategies for the treatment of obesity and metabolic inflammation.
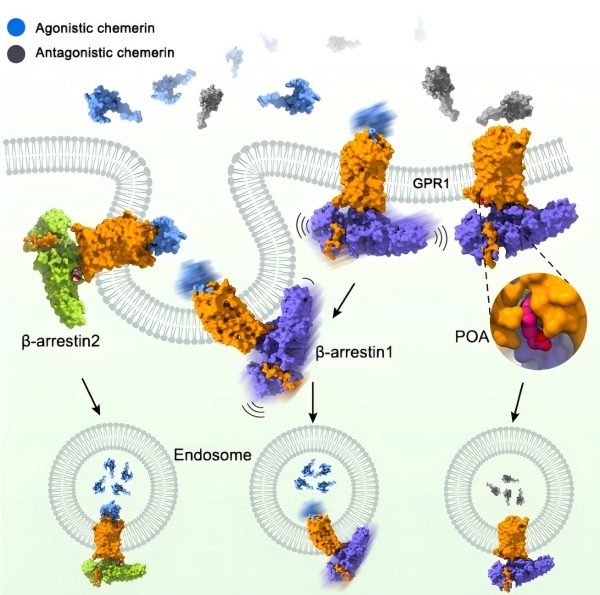
Figure: The new Science study reports the structures of the chemerin receptor GPR1 in complex with different arrestins. GPR1 plays a pivotal role in lipid metabolism and inflammatory responses. The figure shows the structures of GPR1 in complex with β-arrestin 1 or β-arrestin 2. GPR1, β-arrestin 1 and β-arrestin 2 are colored orange, purple and green, respectively. Agonistic and antagonistic chemerin are colored blue and gray, respectively. The palmitoleic acid (POA) that binds to the GPR1–β-arrestin 1 structure is shown as magenta spheres. (Image by Wu Beili’s laboratory at SIMM)
DOI: 10.1126/science.adt8794
Link: https://doi.org/ 10.1126/science.adt8794
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




