Researchers Decode Selective and Dual Blocking of Prostaglandin Receptors EP2 and EP4
Prostaglandin E2 (PGE2), a key lipid mediator, drives inflammation, pain, and cancer progression by activating four G protein-coupled receptors (GPCRs): EP1–EP4. Among these, EP2 and EP4 receptors critically shape the immunosuppressive tumor microenvironment (TME) by inhibiting immune cells and promoting tumor growth. PGE2-EP2/EP4 signaling pathway serves as a key regulatory node connecting active inflammation and immune suppression. Their dual blockade has emerged as a promising strategy for anti-inflammatory and anti-cancer therapies.
In a study published in The EMBO Journal, a research team led by Eric H. Xu (XU Huaqiang) from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences and WU Canrong from Ruijin Hospital of Shanghai Jiao Tong University, using single-particle cryo-electron microscopy (cryo-EM), obtained four inactive structures of EP2 and EP4 receptors bound to selective and dual antagonists.
The obtained structures, included EP2 bound to selective antagonist PF-04418948, EP2 bound to dual antagonist TG6-129, EP4 bound with selective antagonist Grapiprant and EP4 bound with TG6-129, revealed key differences in the binding pockets and the interaction networks, explained why most drugs target one receptor exclusively. The EP2 structures also revealed a unique activation pathway involving transmembrane helices TM1, TM2, TM6, and TM7.
Notably, the researchers revealed a “dual-warhead” binding mode that dual antagonist TG6-129 adopted. One pharmacophore anchored to EP2, while another engaged EP4. This flexible strategy offered a new template for designing balanced EP2/EP4 dual inhibitors.
Taken together, the research fills a critical gap in prostaglandin receptor pharmacology. Prior work focused on active-state structures; these first antagonist-bound complexes provide a blueprint for designing safer, more effective inhibitors.
DOI: 10.1038/s44318-025-00611-0
Link: https://www.embopress.org/doi/full/10.1038/s44318-025-00611-0
Keywords: GPCR antagonist, cryo-EM structure, dual antagonist
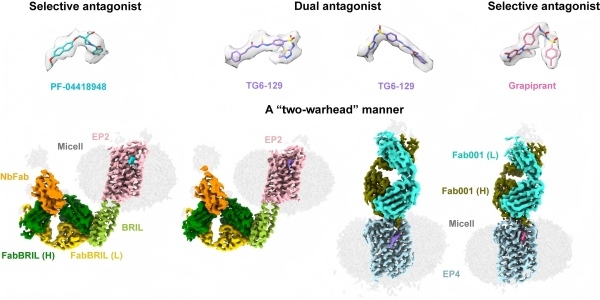
Structures of EP2 and EP4 antagonist complexes. Binding pockets of EP2 and EP4 with TG6-129 revealing the “dual-warhead” mechanism (Image by XU Huaqiang Group)
Contact:
DIAO Wentong
Shanghai Institute of Materia Medica
E-mail: diaowentong@simm.ac.cn




